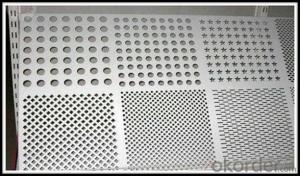
Aluminum Punch Plate
Aluminum Punch Plate Related Searches
Aluminum Push Plate Aluminum Winch Plate Aluminum Metal Plate Aluminum Cooking Plate Aluminum Pressure Plate Aluminum Wall Plate Aluminum Round Plate Aluminum Hot Plate Aluminum Caul Plate Aluminum Paper Plate Polished Aluminum Plate Aluminum Grill Plate Aluminum Kick Plate Aluminum Spinning Plate Aluminum Cheese Plate Aluminum Vin Plate Aluminum Mounting Plate Aluminum Sheet Plate Aluminum Profile Plate Aluminum Backing Plate Aluminum Floor Plate Aluminum Butt Plate Aluminum Square Plate Aluminum Cake Plate Aluminum Motor Plate Aluminum Pie Plate Aluminum Dimond Plate Aluminum Charger Plate Aluminum Plenum Plate Aluminum Joining PlateAluminum Punch Plate Supplier & Manufacturer from China
Aluminum Punch Plate, a type of perforated metal sheet, is widely recognized for its durability and corrosion resistance. This product is crafted from high-quality aluminum, making it an ideal choice for various industrial and commercial applications. Due to its lightweight and strength, it is commonly used in construction, automotive, and aerospace industries, as well as for decorative purposes.The Aluminum Punch Plate finds its application in a multitude of scenarios, such as ventilation systems, filtration systems, and architectural facades. Its versatility allows for customization in terms of hole patterns, sizes, and configurations, catering to specific requirements of different projects. This product is also favored for its ease of installation and maintenance, contributing to its popularity in various sectors.
Okorder.com stands as a prominent wholesale supplier of Aluminum Punch Plate, boasting a vast inventory that caters to the diverse needs of customers. With a commitment to quality and customer satisfaction, Okorder.com ensures that the Aluminum Punch Plate supplied meets the highest industry standards, making it a reliable choice for businesses and projects seeking a durable and efficient solution.
Hot Products