Cyanuric Acid Lead Granular Tablets Powder Standrd Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 1800 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Cyanuric Acid
The Structure of Cyanuric Acid Descriptions:
Trade Name: Isocyanuric Acid
Other name: Cyanuric Acid; 1,3,5-Triazine-2,4,6-triol
Uses: Bleaches and sanitisers.
Formula: C3H3N3O3
Molecular Weight: 129.07
CAS NO.: 108-80-7
Main Features of Cyanuric Acid:
White powder, granular or colored tablet form, non-toxic and odorless
Full experience of large numbers containers loading in Chinese sea port
Fast shipment by reputed shipping line
Packing with pallet as buyer's special request
Best service after shipment with e mail
Cargoes together with container sales seervice available
Full experience for Canada & Japan export
Cargoes photo before and after loading into container
Raw materials from chinese origin
Cyanuric Acid Specification:
| ITEM | SPECIFICATION | RESULT | |||
| Content | ≥98.5% | 98.64% | |||
| Moisture | ≤0.5% | 0.11% | |||
| PH value | 4.0-4.5 | 4.26 | |||
| Fe2+ | ≤15ppm | 7.5ppm | |||
| NH4+ | ≤200ppm | 97ppm | |||
| Ash | ≤0.1% | 0.05% | |||
| Insoluble matter in DMF | ≤0.3% | 0.25% | |||
| Appearance | White crystalline power | White crystalline power | |||
| Mesh number | 95% pass 80 mesh | 95% pass 80 mesh | |||
| White degree | ≥89 | 90.5 | |||
| Conclusion: | The product complies with the standard above. | ||||
Cyanuric Acid Packing:
in 25kg, 1000kg bag for powder
in 25kg plastic bag or 50kg PE drums for granular

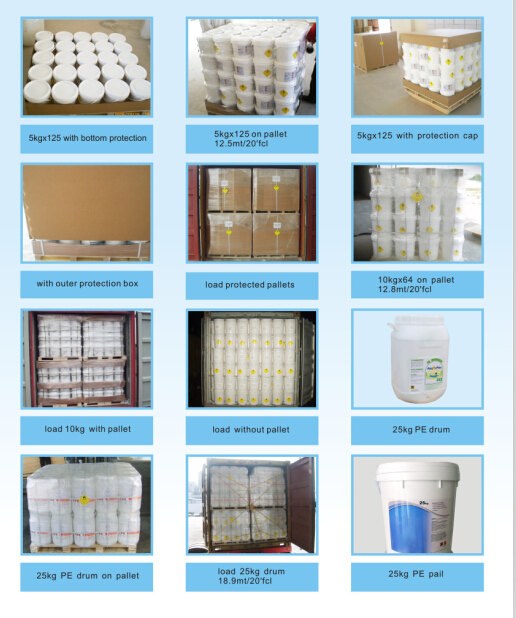

Service we can provide:
1. Mixed container, we can mix different items in one container.
2. Quality control, before shipment, free sample for test. after shipment, keep sample for 3 years
3. Prompt shipment with professional documents
4. Packing as your request, with photo before shipment
Professional Loading:
1.We will provide you with professional loading
2.We have one team supervise uploading the materials. We will check the container, the packages
3.Every step, taking pictures and make records.
4.we will make a complete Loading Report for our customer of each shipment
- Q: Why the amount of catalyst is too small will make the chemical reaction rate slowed down
- Can significantly change the reaction rate and its own chemical properties and quantity in the reaction before and after the basic material unchanged. The catalyst has a positive catalyst (i.e., accelerates the reaction rate) and a negative catalyst (i.e., reduces the reaction rate), and generally does not specifically refer to both the positive catalyst.
- Q: What is a catalyst and how does it make a reaction go faster?
- a catalyst is a substance used to increase and decrease the rate of a chemical reaction....it makes the reaction go faster due to chemical changes it may hav certain characteristics which help in makingthe reaction fast
- Q: Chemical questions: "CO2 and H2 in the catalyst conditions have a reaction, the reaction of the chemical equation is"
- CO2 + H2 = CO + H2O (conditions: catalyst, generally requires heating, and reversible)
- Q: Where are they good catalysts and why?? THanks!
- A catalyst is a substance that speeds up a chemical reaction by providing an alternate reaction pathway with a lower activation energy. Catalysts appear not to take part in the reaction. Frequently, catalysts are not very reactive. Acids and bases, on the other hand, are very reactive. Acids (as H+) and bases ( as OH-) sometimes function as catalysts in some organic reactions. They appear to be catalysts because in the course of the mechanism H+ or OH- is regenerated.
- Q: The chemical reaction equation of methanol heating and oxygen in the presence of catalyst
- Catalytic oxidation of formaldehyde
- Q: What is the difference between a catalyst and an inducer in a chemical reaction?
- The catalyst does not participate in the reaction, but only the carrier of the reaction; the inducer will participate in the reaction
- Q: Just something I've always wondered about...
- there are so much catalysts made up of transition metals. because one of the characteristics of transition metals is can be made to catalyst. one of the catalyst that is mostly used is iron fillings which is used as catalyst to make ammonia from nitrogen gas and hydrogen gas.
- Q: how a catalyst can provide a new route in forming the product?
- a catalyst does not provide a new route. it just lowers something called the energy of activation of the reaction. this makes some changes in the thermodynamics and kinetics of the reaction
- Q: What are the catalysts?
- The catalyst is a substance that can change the rate of the reaction without changing the standard of the reaction Gibbs free, according to the definition of the International Pure and Applied Chemistry (IUPAC) in 1981, Enthalpy change. This action is called catalysis. The reaction involving the catalyst is a catalytic reaction. The catalyst will induce a chemical reaction to change, causing the chemical reaction to become faster or slower or to undergo a chemical reaction at a lower temperature The catalyst is also known as a catalyst in industry, and the composition, chemical properties and quality of the catalyst itself do not change before and after the reaction;
- Q: If possible can anyone give me information on the active site, substrates, products, and the energy of activation as part of the answer?Responses greatly appreciated! Thankss! 10pts to best answer!
- To make it simple unlike the dude above me...enzymes (biological catalysts) lower the activation energy, which speeds up the reaction. EVERY reaction needs a little boost of energy--the activation energy--and enzymes lower that.
Send your message to us
Cyanuric Acid Lead Granular Tablets Powder Standrd Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 1800 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords
























