Industrial Solid Amino Trimethylene Phosphonic Acid
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 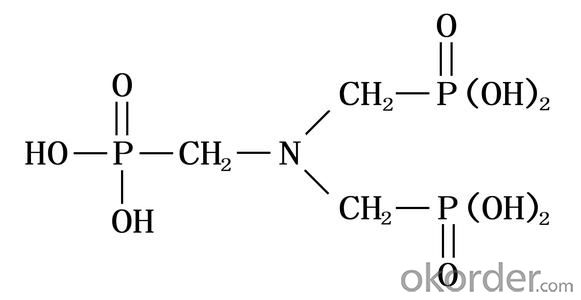
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q:Effect of Catalyst on Chemical Reaction Rate
- The catalyst can only change the rate of chemical change (faster or slower), does not change its own quality and chemical properties, nor does it change the amount of reaction product.
- Q:If possible can anyone give me information on the active site, substrates, products, and the energy of activation as part of the answer?Responses greatly appreciated! Thankss! 10pts to best answer!
- To make it simple unlike the dude above me...enzymes (biological catalysts) lower the activation energy, which speeds up the reaction. EVERY reaction needs a little boost of energy--the activation energy--and enzymes lower that.
- Q:Can Cuo react as a catalyst with H2O2, does its quality and chemical properties change?
- 2H2O2 (CuO catalyzed) ====== 2H2O + O2 ↑
- Q:How does the catalyst affect chemical balance? Why the catalyst has no effect on the chemical equilibrium, for v-t diagram
- The mechanism of the catalyst is to affect the reaction activation energy in the reaction. The positive catalyst reduces the activation energy required for the reaction and increases the proportion of the activated molecules, thus reducing the reaction time.
- Q:How does active charcoal catalyze in some chemical reactions?
- It is a carrier, because its particles are small (micron level, nano-level), has a relatively large surface area, can be loaded on the catalyst to provide more reaction sites. Although called activated carbon, but its catalytic aspects of the relevant business reports.
- Q:What are the catalysts?
- Some chemical reactions are not only the only catalyst, such as potassium chlorate can be thermally decomposed to catalyze the presence of magnesium oxide, iron oxide and copper oxide, etc. The definition of the chemical reaction can change the chemical reaction rate of other substances in the chemical reaction , And its own quality and chemical properties before and after the reaction did not change the material called catalyst, also known as catalyst. Catalyst in the role of chemical reaction called catalysis
- Q:Is the catalyst used in the starch phosphate reaction
- (Cat1, cat2, cat4 and cat5) in the presence of terephthalic acid,
- Q:What is the catalyst called?
- There are three types of catalysts, which are homogeneous catalysts, heterogeneous catalysts and biocatalysts
- Q:Hydrogen and nitrogen in the high temperature and pressure and catalyst conditions for the synthesis of ammonia chemical equation
- 3H2 + N2 = 2NH3 conditional catalyst
- Q:In the catalyst and light conditions to break down the water to get the chemical equation of hydrogen
- 2H2O = (light or catalyst) 2H2 ↑ + O2 ↑
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Industrial Solid Amino Trimethylene Phosphonic Acid
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
























