Sodium Dichloroisocyanurate SDIC For Water Treament
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 9000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Sodium Dichloroisocyanurate SDIC
Introduction:
SDIC White powder or grain with chlorine odor . It is a strong oxidant and chlorate agent and can dissolved in water easily . Its aqueous solution
assumes weak acidity and the active chlorine in its dry products lose little when it is stored for a long time at the atmospheric temperature .
Specification:
Chemical Name | Sodium Dichloroisocyanurate | |
Molecular Formula: | C3O3N3HCL2NA | |
Molecular Weight: | 220.96 | |
CAS Number: | 2893-78-9 | |
Product | 60% | 56% |
Available chlorine(%,min) | 60 | 56 |
Moisture content(% max) | 5 | 8 |
PH Value(1% solution) | 6-7 | 6-7 |
Particles Size:
Mesh | 5~8 | 8~30 | 20~40 | 20~60 |

Main usage:
this products can effectively kill various germs, fung uses and viruses, specially A&B type hepatitis viruses. It is effective on killing algae,
decolorizing cleaning water or bleaching .It can be widely used for epidemic prevention, livestock farming , industry and agriculture.

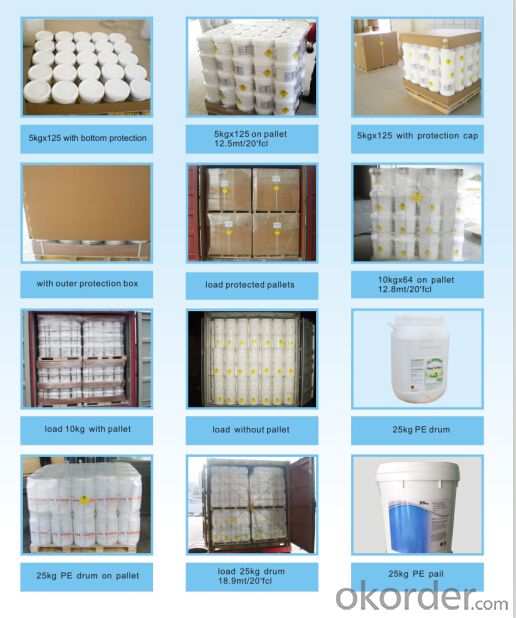
Package:
50KG PLASTIC DRUMS/ FIBER DRUMS.25KG PLASTIC DRUMS/FIBER DRUMS. 1000KG BIG BAGS. Or any other packages
suggest by customers.

- Q: High school knowledge __ teacher do not know right!
- Nothing to do, but with the percentage of activated molecules, is proportional to
- Q: Chemistry is often said that the catalyst can change the material reaction rate, rate and speed What is the difference
- The rate is only size, no direction, speed both size and direction
- Q: in acid-catalyzed reaction,there are some books show the acid catalyst as H+ and there are some show it as H3O+ .Are they the same?
- H+ is the ion contained in acids.... When acids are dissolved in water (H+)+(H2O)=H3O+ Both are the same......
- Q: Like biological and industrial reations. Thanks.
- Reactions that have high Activation Energy need catalysts to speed up reactions. These reactions aren't spontaneous since the reactants do not have enough energy to overcome the activation energy barrier. Catalysts are compounds that speed up reactions by providing an alternative pathway for the reaction. It is a common misconception that catalysts lower the activation energy. It doesn't actually lower the activation energy, instead it provides an alternative pathway with lower activation energy. For example, breakdown of hydrogen peroxide happens in nature but, relatively slowly. When you add a little bit of manganese dioxide, the breakdown happens a lot faster. Another example is, breakdown of glucose in the body. It is facilitated by an enzyme called amylase (or carbohydrase). An industrial example is the use of vanadium pentoxide (V2O5) in the Contact process, where SO2 is converted to SO3 in the presence of V2O5. Hope that helps!
- Q: Chemical Glossary: Catalyst
- The catalyst is a substance that can change the rate of the reaction without changing the standard of the reaction Gibbs free, according to the definition of the International Pure and Applied Chemistry (IUPAC) in 1981, Enthalpy change. This effect is called catalysis. The reaction involving the catalyst is a catalytic reaction.
- Q: Why the catalyst is required to have a large surface area and a rich pore structure
- In order to speed up the absorption, speed up the reaction rate
- Q: Effect of Catalyst on Chemical Reaction Rate
- The catalyst can only change the rate of chemical change (faster or slower), does not change its own quality and chemical properties, nor does it change the amount of reaction product.
- Q: A substance involved in chemical reflection, but reflects the quality of the material before and after the change, you say it is a catalyst?
- A catalyst is a substance that accelerates the reaction rate of other reactions and whose quality and chemical properties do not change. Sometimes the catalyst can also participate in the reaction. The elemental iodine in the title is the catalyst for the decomposition of hydrogen peroxide.
- Q: Chemical "catalyst can speed up the chemical reaction rate of other substances," this sentence right?
- Wrong, the catalyst is divided into two kinds, one is to speed up the chemical reaction speed, and the other is the opposite
- Q: In the chemical reaction will have to use the catalyst reaction, such as H2O2 === (MnO2) H2O + O2 ↑, then the catalyst in the end to participate in the reaction (that is, the catalyst itself is the reactant) If so, why are some of these substances in the reaction (these substances refer to the catalyst) in the reaction after the quality and nature of the change does not change?
- The composition, chemical properties and quality of the catalyst itself do not change before and after the reaction; its relationship with the reaction system is as highly selective (or specific) as the relationship between the lock and the key. A catalyst does not catalyze all chemical reactions. For example, manganese dioxide catalyzes the thermal decomposition of potassium chlorate, accelerates the reaction rate, but does not necessarily have a catalytic effect on other chemical reactions. Some chemical reactions are not only the only catalyst, such as potassium chlorate can be thermally decomposed to catalyze the presence of magnesium oxide, iron oxide and copper oxide and so on.
Send your message to us
Sodium Dichloroisocyanurate SDIC For Water Treament
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 9000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches






























