Cyanuric Acid Granular,Tablets, Powder National Standard Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 22 m.t.
- Supply Capability:
- 1800 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Cyanuric Acid
General Descriptions:
Trade Name: Isocyanuric Acid
Other name: Cyanuric Acid; 1,3,5-Triazine-2,4,6-triol
Uses: Bleaches and sanitisers.
Formula: C3H3N3O3
Molecular Weight: 129.07
CAS NO.: 108-80-7
Appearance: White powder, granular or colored tablet form, non-toxic and odorless
Specification:
| ITEM | SPECIFICATION | RESULT | |||
| Content | ≥98.5% | 98.64% | |||
| Moisture | ≤0.5% | 0.11% | |||
| PH value | 4.0-4.5 | 4.26 | |||
| Fe2+ | ≤15ppm | 7.5ppm | |||
| NH4+ | ≤200ppm | 97ppm | |||
| Ash | ≤0.1% | 0.05% | |||
| Insoluble matter in DMF | ≤0.3% | 0.25% | |||
| Appearance | White crystalline power | White crystalline power | |||
| Mesh number | 95% pass 80 mesh | 95% pass 80 mesh | |||
| White degree | ≥89 | 90.5 | |||
| Conclusion: | The product complies with the standard above. | ||||
Packing:
in 25kg, 1000kg bag for powder
in 25kg plastic bag or 50kg PE drums for granular

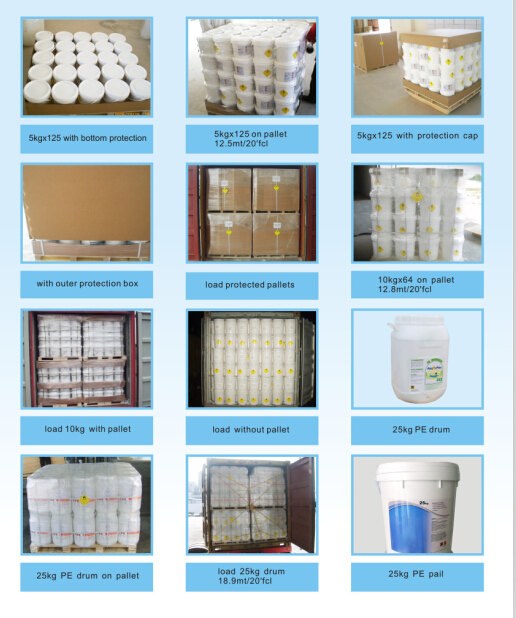

Storage:
kept in a light-proof,well-colsed,dry and cool place.
Service we can provide:
1. Mixed container, we can mix different items in one container.
2. Quality control, before shipment, free sample for test. after shipment, keep sample for 3 years
3. Prompt shipment with professional documents
4. Packing as your request, with photo before shipment
Our Advantages:
Full experience of large numbers containers loading in Chinese sea port
Fast shipment by reputed shipping line
Packing with pallet as buyer's special request
Best service after shipment with e mail
Cargoes together with container sales seervice available
Full experience for Canada & Japan export
Cargoes photo before and after loading into container
Raw materials from chinese origin
Professional Loading:
1.We will provide you with professional loading
2.We have one team supervise uploading the materials. We will check the container, the packages
3.Every step, taking pictures and make records.
4.we will make a complete Loading Report for our customer of each shipment
- Q: Hydrogen and oxygen in the role of the catalyst can do the chemical formula of aviation fuel?
- 2H2 + O2 = catalyst = 2H2O
- Q: What is the similarity between enzymes and general chemical catalysts?
- (1) The enzyme is the same in many respects as a biocatalyst and a general catalyst, such as a small amount and a high catalytic efficiency. As with the general catalyst, the enzyme can only change the rate of chemical reaction and does not change the equilibrium of the chemical reaction It is possible to catalyze the activation of a large number of substrates in a short time and to reflect the high efficiency of enzyme catalysis.The enzyme can reduce the activation energy of the reaction (activation) (△ G) during the reaction, but the reaction rate is accelerated and the reaction time is reduced, but the equilibrium constant is not changed. (2) However, the enzyme is a biological macromolecule (1) Enzyme-catalyzed high efficiency: The catalytic effect of the catalyst can increase the reaction rate by 10 ^ 6 ~ 10 ^ 12 times, which is at least several times higher than that of the conventional catalyst. (2) The enzyme catalyst Highly specificity: including specificity of response, substrate specificity, chirality specificity, geometric specificity, etc., that an enzyme can only act on a certain class or a specific substance. Bond, ester bond, peptide bond and so on can be catalyzed by acid-base hydrolysis, but the hydrolysis of these chemical bonds are different, respectively, the corresponding glycosidase, esterase and peptidase, that is, they were specific (3) enzymatic reaction conditions are mild: enzymatic reaction is generally carried out in aqueous solution of pH = 5 ~ 8, the reaction temperature range is 20 ~ 40 ℃
- Q: in acid-catalyzed reaction,there are some books show the acid catalyst as H+ and there are some show it as H3O+ .Are they the same?
- Short=yes they are. Long version Traditionally, acids were defined to be compounds that produce H+ ions when dissoved in water (Arrhenius theory). But this definition is limited to acids that can be dissolved in water. Br?nsted-Lowry then formed a definition which states that acids are compounds which donates a protons or H+ ions. If u think about it, a H+ ion is practically a proton. a proton with no electron outer shell is far too reactive to stay in its current state. thus it will form a bond with H2O to form H3O+ Because of this, acid catalysts are supose to be H3O+ instead of H+. But since it is more convinient to use H+, the form of writing H+ remained instead....... and yes...... the scientists were lazy......
- Q: Like biological and industrial reations. Thanks.
- Reactions that have high Activation Energy need catalysts to speed up reactions. These reactions aren't spontaneous since the reactants do not have enough energy to overcome the activation energy barrier. Catalysts are compounds that speed up reactions by providing an alternative pathway for the reaction. It is a common misconception that catalysts lower the activation energy. It doesn't actually lower the activation energy, instead it provides an alternative pathway with lower activation energy. For example, breakdown of hydrogen peroxide happens in nature but, relatively slowly. When you add a little bit of manganese dioxide, the breakdown happens a lot faster. Another example is, breakdown of glucose in the body. It is facilitated by an enzyme called amylase (or carbohydrase). An industrial example is the use of vanadium pentoxide (V2O5) in the Contact process, where SO2 is converted to SO3 in the presence of V2O5. Hope that helps!
- Q: The concept of catalyst in high school chemistry
- In the chemical reaction can change the chemical reaction rate of other substances, and its own quality and chemical properties before and after the reaction did not change the material called catalyst (also known as catalyst).
- Q: Why can't catalysts make an unfavorable reaction favorable?Can anyone give me a relatively simple explanation for this?Thank you so much in advance!
- Catalysts, as enzymes, only change the activation energy (the energy the compound needs to gain to transform into products), they don't change the Gibbs energy values of reactants nor products. Therefore, if the delta G of the reaction is positive, it'll still need free energy to complete. They make a reaction complete faster than in normal conditions, but don't change the actual possibility for that reaction to happen. In the human body, a lot of reactions of catabolism have a positive G value and these reactions needs to get energy from other coupled reactions that have a negative value, so the total value is still negative. Many of them use hydrolysis of ATP to provide that energy, as its hydrolysis is about -30 kJ/mol in physiological conditions. I don't know what class you're in to ask this question, so can't really know if this answer is too simple or complicated for u... sorry in advance! Jo?l
- Q: Is the catalyst in the chemical reaction better?
- The efficiency of the catalyst is very high, as long as a little bit on it, with too much in addition to increase the cost of meaningless. Positive catalyst can increase the rate of several thousand times. Fast ... I know there will be about a thousand years shortened to 1 second. There are slowdown in the catalyst, people taught not learned. Other versions of the textbook is not clear. Should not learn.
- Q: about 1-3 sentences on this will do thank you
- a catalyst is something that makes a reaction go faster than it normally would. An enzyme is a catalyst; it has all the parts for the reaction on it and help organic materials break down or transfer energy or whatever reaction it needs.
- Q: Manganese dioxide can be used as a catalyst for various chemical reactions
- The reactor may be a reactant,
- Q: If possible can anyone give me information on the active site, substrates, products, and the energy of activation as part of the answer?Responses greatly appreciated! Thankss! 10pts to best answer!
- To make it simple unlike the dude above me...enzymes (biological catalysts) lower the activation energy, which speeds up the reaction. EVERY reaction needs a little boost of energy--the activation energy--and enzymes lower that.
Send your message to us
Cyanuric Acid Granular,Tablets, Powder National Standard Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 22 m.t.
- Supply Capability:
- 1800 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords





















