Cyanuric Acid Powder Standard Quality From China Factory
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 1800 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Cyanuric Acid
General Descriptions:
Trade Name: Isocyanuric Acid
Other name: Cyanuric Acid; 1,3,5-Triazine-2,4,6-triol
Uses: Bleaches and sanitisers.
Formula: C3H3N3O3
Molecular Weight: 129.07
CAS NO.: 108-80-7
Appearance: White powder, granular or colored tablet form, non-toxic and odorless
Specification:
| ITEM | SPECIFICATION | RESULT | |||
| Content | ≥98.5% | 98.64% | |||
| Moisture | ≤0.5% | 0.11% | |||
| PH value | 4.0-4.5 | 4.26 | |||
| Fe2+ | ≤15ppm | 7.5ppm | |||
| NH4+ | ≤200ppm | 97ppm | |||
| Ash | ≤0.1% | 0.05% | |||
| Insoluble matter in DMF | ≤0.3% | 0.25% | |||
| Appearance | White crystalline power | White crystalline power | |||
| Mesh number | 95% pass 80 mesh | 95% pass 80 mesh | |||
| White degree | ≥89 | 90.5 | |||
| Conclusion: | The product complies with the standard above. | ||||
Packing:
in 25kg, 1000kg bag for powder
in 25kg plastic bag or 50kg PE drums for granular

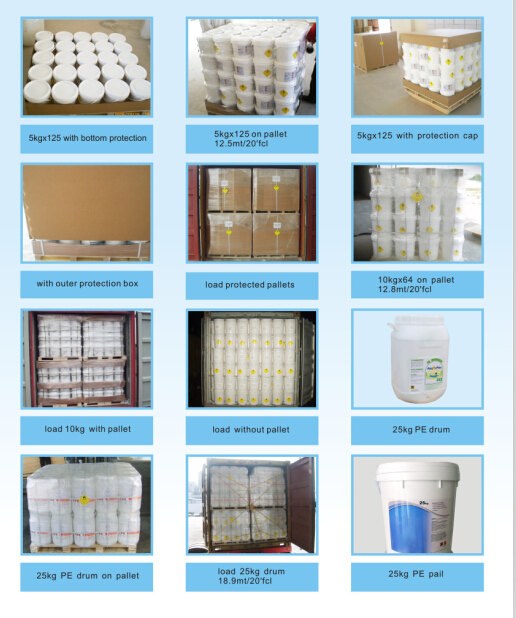

Storage:
kept in a light-proof,well-colsed,dry and cool place.
Professional Loading:
1.We will provide you with professional loading
2.We have one team supervise uploading the materials. We will check the container, the packages
3.Every step, taking pictures and make records.
4.we will make a complete Loading Report for our customer of each shipment
- Q: What are the pharmaceutical manufacturing companies now using PT / AL_203 catalysts?
- I have a friend working in Hunan, inquire, as if the piece of PT has the most advanced equipment ,,, you can hit 114 inquiries ~!
- Q: High school knowledge __ teacher do not know right!
- Nothing to do, but with the percentage of activated molecules, is proportional to
- Q: How do I write about the ion equation?
- 4NH3 + 5O2 == 4NO + 6H2O
- Q: Will the chemical catalyst not reduce that?
- Why is it done? Although the catalyst does not react chemically, the catalyst itself is deteriorated and is not always used
- Q: What is chemical adsorption and its relationship with heterogeneous catalysis
- The catalytic cycle includes five steps: diffusion, chemical adsorption, surface reaction, desorption and reverse diffusion.The chemical adsorption is an important part of the heterogeneous catalysis process, and the adsorption of the reactants on the catalyst surface,
- Q: How does active charcoal catalyze in some chemical reactions?
- Activated carbon is generally in the catalytic reaction to do more carriers, has not yet seen its catalyst to report.
- Q: Does anybody have any tips or references I can go to for this? I'm writing a novel and the main character wants to be a catalyst; the story isn't about him and his journey, so much as the effects on everyone around him that come about simply because of him being there and being who he is. How would I go about doing this, and doing it well?
- Ways to be a catalyst: By his behaviour: - He is a good listener. People use him as a sounding board and make important decisions as a result. - He is indiscreet and inadvertently reveals people's secrets and back-sniping comments. - He is a ****-stirring gossip. - He is a home-wrecking Lothario. - He is wise and gives good advice. - He is a hooligan who ruins businesses with vandalism, costs people their jobs and ruins relationships by beating people up. - He is a manipulative, blackmailing bastard who tries to turn every woman into a prostitute and con every man out of his money. - He is a charlatan who preaches nonsense about religion, health and business investments. By effortlessly influencing other people's behaviour: - He is famous and people try to impress him wherever he goes. - He has cancer or a disabling war wound. People admire and pity him and are shocked by his PTs mood wings. - He is gay, Muslim or a suspected paedophile and people want to persecute or cure him. - He is destitute. People argue amongst themselves over whether it's because he's lazy, has bad karma or there but for the grace of God go I. - He really looks like Jesus, Buddha or Santa and the sight of him makes people contemplate their Humanist values, coming to various conclusions. Perhaps you can write about his reputation; how he earned it, how it precedes him and provokes prejudices that he sometimes confirms or disproves. Use a detached, omniscient God-narrator. (Pretend Morgan Freeman's reading the audiobook.) Alternatively perhaps he is not a catalyst but a neurotic voyeur, fascinated by the minutiae of other people's lives so that the mundane appears tumultuous. He is not influencing people but your account of the changes in people's lives are centred on his observation of them.
- Q: Word editor when playing chemical equation = with the above conditions or how to adjust the size of the catalyst, how to make it centered,
- Open the word - insert - object - WPS3.0 formula - and then select the "label arrow template", you can add a catalyst.
- Q: Manganese dioxide can be used as a catalyst for various chemical reactions
- The reactor may be a reactant,
- Q: Can you describe at least 4 ways a catalyst can lower the activation energy of a reaction?
- To see how a catalyst accelerates the reaction, we need to look at the potential energy diagram shown below which compares the non-catalytic and the catalytic reaction. For the non-catalytic reaction, the figure is simply the familiar way to visualize the Arrhenius equation: the reaction proceeds when A and B collide with succificient energy to overcome the activation barrier. The change in Gibbs free energy between reactants, A + B, and the product P is delta G. The catalytic reaction starts by bonding of the reactants A and B to the catalyst, in a spontaneous reaction. Hence, the formation of this complex is exothermic and the free energy is lowered. There then follows the reaction between A and B while they are bound to the catalyst. This step is associated with an activation energy; however, it is significantly lower than that for the uncatalyzed reaction. Finally, the product P seperates from the catalyst in an endothermic step. The energy diagram illustrates 4 ways the catalyst works : The catalyst offers an alternative path for the reaction that is energetically more favorable The activation energy of the catalytic reaction is significantly smaller than that of the uncatalyzed reaction; hence the rate of the catalytic reaction is much larger The overall change in free energy for the catalytic reaction equals that of the uncatalyzed reaction. Hence, the catalyst does not affect the equilibrium constant for the overall reaction. A catalyst cannot change the thermodynamics of a reaction but it can change the kinetics. The catalyst accelerates both the forward and the reverse reaction to the same extent. In other words, if a catalyst accelerates the formation of product P from A and B, it will do the same for the decomposition of P into A and B.
Send your message to us
Cyanuric Acid Powder Standard Quality From China Factory
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 1800 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
























