Large Sheets of Aluminum Coil 5754 5005 8079 8011 1050 1060 for Cans
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 10000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Specification
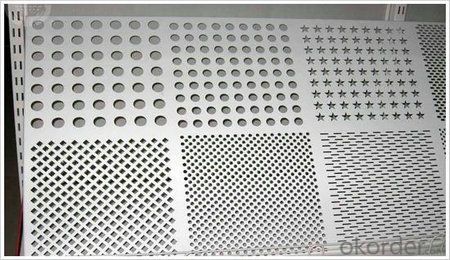
Pattern: orange peel, aluminum sheet5 bar,half a ball,diamond,compass shape
1.Orange peel aluminum sheet/coil
1)Alloy :1060 1100 1050 3003 3004 3105 5052
2)Temper :0 H114 H24 H32
3) Specification thickness:0.2mm-3.0mm
width:50mm-1300mm
Length: according your request
4)Application: Refrigeration. Air-conditioner. keep warm well such as pipe ,tank
5)Surface: No crack,canker, hole
2. aluminum sheet5 bar/coil
1)Alloy :1060 1100 1050 3003 3004 3105 5052 5754 5083 6061 6063 6082
2)Temper: O H114H194 T6
3) Specification: Thickness:1.0mm-10mm
Width:50mm-2000mm
Length: according your request
4)Application: the main function is anti-skidding,widely used in automobile manufacturing,shipbuilding,machine manufacturing,elerator,workshop
5)Surface: No crack,canker, hole
3.Compass shape aluminum sheet/coil
1)Alloy:1060 1100 1050 3003 3004 3105 5052 5754 5083 6061 6063 6082
2)Temper: O H114 H194 T6
3) Specification: thickness:1.0mm-10mm
width:50mm-2000mm
Length: according your request
4)Application: the main function is anti-skidding,widely used in automobile manufacturing,shipbuilding,machine manufacturing,elerator,workshop
5)Surface: No crack,canker, hole
4.Diamond aluminum sheet/coil
1)Alloy:1060 1100 1050 3003 3004 3105 5052 5754 5083 6061 6063 6082
2)Temper: O H114 H194 T6
3) Specification: Thickness:1.0mm-10mm
Width:50mm-2000mm
Length: according your request
4)Application: building industry,packing,decorative
5)Surface: No crack,canker, hole
4) Delivery time: 15-20 days after confirming order.
For all above:
1) Minimum order quantity: 5 tons per size.
2) The term of payment: T/T, irrevocable L/C at sight.
3) Packing: Export standard, waterproof wooden,craft,plywood pallet,etc.




- Q: If not, where can i buy some aluminum?
- Elemental aluminum is far too reactive to exist in nature therefore it must be found in a compound with something else. Aluminum foil is actually made up of aluminum alloys. An alloy is a solid metal created by combining at least one metal with at least one other chemical. One classic example of an alloy is steel. Steel is mostly iron bonded to carbon atoms throughout it's structure. While aluminum foil is technically 92-99% aluminum, it is not pure (elemental) aluminum. Other chemicals that may be found in the alloys are typically other metals: copper, magnesium, manganese, silicon, and zinc. Other nonmetal chemicals may be found aswell like: oxygen (oxides), sulfates (SO4), Phosphates (PO4), and chlorides. You cannot buy elemental aluminum however since aluminum foil is generally 92-99% aluminum it is still a great source if you need aluminum as a reagent. Just for added trivia, the most common form of aluminum on earth is as the mineral Bauxite which is a hydrate of aluminum (Various forms of aluminum bonded to a hydroxide (OH) group such as Al(OH)3, and Al(OH)).
- Q: Can aluminum sheets be used for window frames?
- Yes, aluminum sheets can be used for window frames. Aluminum is a popular choice for window frames due to its durability, lightweight nature, and resistance to corrosion. Aluminum frames offer excellent strength and stability, and they can be easily customized to fit any window size or shape. Additionally, aluminum frames require minimal maintenance and can be painted or anodized to match any desired color or finish. Overall, aluminum sheets are a versatile and reliable material for constructing window frames.
- Q: Can aluminum sheet be used for food contact applications?
- Yes, aluminum sheet can be used for food contact applications. Aluminum is a safe and commonly used material for food packaging and cooking utensils due to its non-toxic properties, resistance to corrosion, and ability to maintain food quality and freshness.
- Q: How is aluminum sheet made?
- Aluminum sheet undergoes a rolling process to be manufactured, known as rolling. This process entails passing a preheated aluminum ingot through a succession of rolling mills. The initial step involves heating the ingot to a specific temperature to enhance its malleability. Once heated, the ingot is then fed through a sequence of rolling mills, wherein it is continuously pressed and stretched to reduce its thickness and increase its length. The rolling mills consist of a pair of rotating cylinders with grooves on their surfaces. As the ingot passes through these cylinders, it undergoes compression and elongation, resulting in a thinner and longer sheet. This process is repeated multiple times, with each pass further reducing the sheet's thickness. To maintain the desired thickness and dimensions, the sheet is periodically subjected to annealing. This process involves heating the sheet to a specific temperature and gradually cooling it. Annealing helps alleviate internal stresses and ensures the sheet retains its desired properties. Once the desired thickness is achieved, the aluminum sheet is cooled, trimmed, and either cut into specific lengths or rolled into coils for further processing or distribution. The final product is a flat, thin, and versatile sheet of aluminum that finds applications in various industries, including construction, automotive, packaging, and electrical appliances.
- Q: Aluminum and oxygen gas react to produce aluminum oxide
- Aluminium oxide is Al2O3, so there are 3 oxygen atoms for every 2 aluminium atoms. Divide 75 by the relative atomic mass of oxygen (15.9994). That is proportional to the number of oxygen atoms. Then divide by 3 and multiply by 2. This gives a number proportional to the number of aluminium atoms. Then multiply this by the relative atomic mass of aluminium (26.981529) to give the mass of aluminium required in grams.
- Q: im wondering what happens if you MIG weld aluminum with the correct wire but without using a shielding gas? Would it just be extremely messy or does there HAVE to be an inert gas flowing for it to bond?thanks
- welding reaches high enough heats that the oxygen and hydrogen, and nitrogen that naturally occure in our atmosphere will combine and or otherwise interfere with the re-solidification of the molten metal. This will completely destroy the new molecular structure and there will be no strength in the new metal. All welding uses some form of preventing the atmospheres oxygen,nitrogen, and hydrogen from effecting the alloy structure. Other elements can also be combined in the chemical reaction if your environment or your project are not clean. All metals oxidize on their surface. That means combine with oxygen. It is the process we call rust when refering to iron which in the case of iron never stops rusting until it rusts away. for the most part all other metals oxidize on their surface and stop. this is what gives their great protection and helps in deciding their use,as with aluminum. You can create environments that will cause thsese other metals to continue to oxidize as with electrolosis, but that is getting more complex. SOO with aluminum it is a good idea to brush the oxidation layer off the surfaces to be welded so this oxidised aluminun does not become a source of contamination in the new weld metal. It is recomended to use a clean stainless steel bristle brush as an iron brush can contaminate. Brushing can generally last for many hours even days but you can see the dull color re appear as it oxidizes over time. Another comon source of oxygen and hydrogen is water. That is what the purpose of low hydrogen electrodes is, though these are not used in aluminum. The military builds the aluminum hulls for the bradley and other vehicles in San Jose partly because the relative humidity is best for mig welding aluminum there. so you can see the concepts can become conflicting. Always use argon with aluminum. Argon can be mixed with small percentages of helium too.
- Q: Would a chemical reaction happen between a piece of copper and a liquid solution containing a compound of aluminum? Why or why not? FIRST BEST ANSWER GETS POINTS!
- No. Copper metal will not reduce aluminum ions to aluminum metal. We can observe that copper is below aluminum in the activity series, which indicates that the Cu + Al3+ reaction is nonspontaneous. The other way round will work, sort-of. Aluminum metal will reduce copper ions. But it takes freshly prepared aluminum metal. Aluminum metal passivates, that is, it reacts with oxygen to form an Al2O3 layer on the surface of the metal. The presence of chloride ion will help provide a clean Al surface.
- Q: How does the thermal conductivity of aluminum compare to other metals?
- Compared to many other metals, aluminum boasts a relatively high thermal conductivity. It is often hailed as one of the foremost heat conductors among common metals. With a thermal conductivity of approximately 205 watts per meter kelvin (W/m·K), aluminum surpasses copper (about 401 W/m·K) and even silver (about 429 W/m·K). As a result, aluminum excels at efficiently transferring heat, making it a widely favored option for heat sinks, radiators, and various applications necessitating effective heat dissipation. Nonetheless, it is worth mentioning that certain metals, such as diamond and graphene, surpass aluminum in terms of thermal conductivity.
- Q: This question asks if aluminum sheets are suitable for outdoor use in environments with high temperatures.
- <p>Yes, aluminum sheets can be used for outdoor applications even in high temperatures. Aluminum has a high melting point of around 660掳C (1220掳F), making it resistant to heat. It also has good thermal conductivity, which allows it to dissipate heat quickly. However, it's important to note that prolonged exposure to high temperatures can cause aluminum to expand and potentially warp. For outdoor applications, especially in high-temperature environments, it's recommended to use aluminum alloys designed for such conditions to ensure durability and performance.</p>
- Q: Hi guys. I was just wondering if you can give me some tips or information on what you know about painting on metal. The metal that is of concern is aluminium and I would like to put a stencil over this aluminium and just blast it with a can of krylon. Of course...this aluminium piece is actually part of the housing for my cell phone so doing it like this would probably not be right and the paint would probably wear out. I am mainly interested in finding out what kind of paint to use, what tools i need, and how to achieve an extremely! durable! matte finish because this phone is thrown around and going in and out of my pocket the paint will have to withstand getting rubbed down everyday by my pockets.That was a very long question, thanks for bearing with me and thanks in advance for those who provided me with an answer/thought. THANKS!
- Spray Painting Aluminum
Send your message to us
Large Sheets of Aluminum Coil 5754 5005 8079 8011 1050 1060 for Cans
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 10000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords


































