Amino Trimethylene Phosphonic Acid Liquid And Solid
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 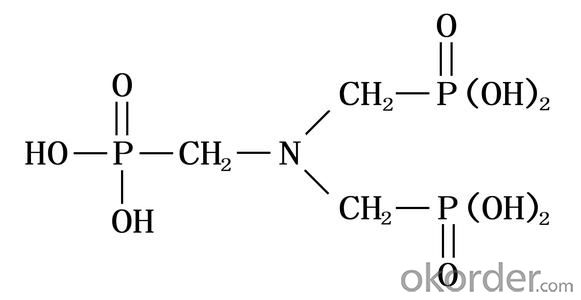
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q: What are the pharmaceutical manufacturing companies now using PT / AL_203 catalysts?
- I have a friend working in Hunan, inquire, as if the piece of PT has the most advanced equipment ,,, you can hit 114 inquiries ~!
- Q: why is palladium/platinum a good catalyst?
- Palladium and platinum can form partial bonds with other molecules. By forming these partial bonds, the bond in the actual molecule gets weaker and weaker and hence, the bond becomes easier to break. Let's say for example a hydrogen molecule. There is a single bond between the 2 hydrogen atoms. Platinum/palladium will form partial bonds with the 2 hydrogen atoms. By doing so, the single bond BETWEEN THE 2 HYDROGEN ATOMS gets weaker and weaker. Hence, a smaller amount of energy is needed to break the bond between the 2 hydrogen atoms (the hydrogen molecule). As the amount of energy needed to overcome the bond between the 2 hydrogen atoms gets smaller, we say the activation energy for the reaction has been reduced. Hence, a greater amount of bonds in hydrogen molecules can be broken in a smaller time, and therefore, we say palladium/platinum has catalysed the reaction.
- Q: How does the catalyst affect chemical balance? Why the catalyst has no effect on the chemical equilibrium, for v-t diagram
- Chemical reaction to reactor molecules to reach a certain amount of energy in order to react, this energy is the activation energy of the reaction. While the material energy in nature can be obtained by probability statistics
- Q: How does the chemical equation calculate the quality of the catalyst?
- Catalyst, the quality of the reaction before and after the same, the same chemical properties.
- Q: What is chemical adsorption and its relationship with heterogeneous catalysis
- The catalytic cycle includes five steps: diffusion, chemical adsorption, surface reaction, desorption and reverse diffusion.The chemical adsorption is an important part of the heterogeneous catalysis process, and the adsorption of the reactants on the catalyst surface,
- Q: The future direction of employment how, in what kind of units to do what work, how the closure rate? The
- Generally in the chemical plant to do engineering design engineers, the past few years, science and engineering graduates generally do not worry about work.
- Q: My chemistry teacher wont tell me because it's in the higher course. And i'm not waiting a whole year to find out. And also, google is being a gimp about it. So thanks a lot if you know, I only have basic chemistry knowledge btw, lumen'ss terms if you can.
- Catalysts facilitate the reaction. They might work in several ways. Here is an example: Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process regenerating the catalyst. The following is a typical reaction scheme, where C represents the catalyst, X and Y are reactants, and Z is the product of the reaction of X and Y: X + C → XC (1) Y + XC → XYC (2) XYC → CZ (3) CZ → C + Z (4) Although the catalyst is consumed by reaction 1, it is subsequently produced by reaction 4, so for the overall reaction: X + Y → Z They might also just increase the surface area, thus speeding up the reaction. Example: Coke looses its fizz over time if left with the cork unscrewed. This is because the HCO3 is released as CO2. If you drop a menthos into the coke, it explodes with CO2, because the methos is full of tiny dents in the surface (thus giving it a massive surface area). (i blatantly copied the first example from the wiki)
- Q: and can you give me an example of it .. please give it in easy terms if you can. thanks
- the compound that allows for a chemical reaction. and example would be The enzyme catalase is the catalyst that allows hydrogen peroxide to break down into water
- Q: Chemical reaction in the presence of catalyst for the reaction are carried out a high life
- Some reactions require some of the catalyst that is not needed
- Q: chemistry subject
- A catalyst is a substance which alters the rate of a chemical reaction but is chemically unchanged at the end of the reaction there are well some of em are 2,2'-Bis(2-indenyl) biphenyl Adams' catalyst Band 3 Cerium(IV) oxide Copper(II) acetate Copper(II) hydroxide Cyclooctadiene rhodium chloride dimer 4-Dimethylaminopyridine Enzyme engineering Faujasite Frustrated Lewis pair Grubbs' catalyst Hopcalite Incipient Wetness Impregnation Lanthanide triflates Lindlar catalyst Mesoporous silicates Methylaluminoxane NOBIN Nickel(III) oxide Noxer block Palladium on carbon Phase transfer catalyst Platinum Polyoxometalate P cont. Post-metallocene catalyst 2-Pyridone Pyrotol catalyst Raney nickel Ribozyme Tetrakis(triphenylphosphine)palladium(... Tetrakis(triphenylphosphine)platinum(0... Triazabicyclodecene Tris(dibenzylideneacetone)dipalladium(... Trost ligand Vanadium(V) oxide Wilkinson's catalyst Ziegler-Natta catalyst
Send your message to us
Amino Trimethylene Phosphonic Acid Liquid And Solid
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches



















