Amino Trimethylene Phosphonic Acid Water Treatment Chemical
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 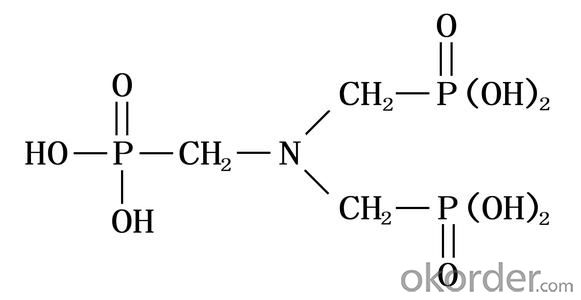
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q: Chemistry GCSE what is a catalyst?
- Enzymes are catalysts in organic and organic strategies. many times catalysts that are used for business or lab reactions are fairly basic compounds. diverse reactions use diverse catalysts, that's because of the various reaction mechanisms. Catalysts take part in a reaction yet end unchanged. Hydrogenation catalysts are factors which includes platinum, nickel and so on. those metals react with the hydrogen on the exterior of them and the different reactant which includes vegetable oil to produce a product. Platinum is is utilized in catalytic converters on automobiles, in spite of the undeniable fact that lead can injury the platinum by potential of blocking off the exterior, subsequently you shouldn't use leaded petrol in automobiles with catalytic converters. Vanadium pentoxide is used to catalyse the reaction of SO2 to SO3 . and so on and so on. So in biology diverse enzymes are required for various reactions, reckoning on the reaction mechanism. Starch hydrolyses to glucose with an enzyme spoke of as ptaylin, cutting-edge in saliva. Proteins choose enterokinase to start the reaction all the way down to amino acids. there are a number of enzymes for various chemical strategies, that shall we not stay to tell the tale without. Animals that graze which includes cows and horses have enzymes cutting-edge of their digestive tract which will ruin down cellulose, we don't, so as that they are in a position to consume grass and so on and extract glucose from it. desire this helps, and not confuses.
- Q: What is the similarity between enzymes and general chemical catalysts?
- (1) The enzyme is the same in many respects as a biocatalyst and a general catalyst, such as a small amount and a high catalytic efficiency. As with the general catalyst, the enzyme can only change the rate of chemical reaction and does not change the equilibrium of the chemical reaction It is possible to catalyze the activation of a large number of substrates in a short time and to reflect the high efficiency of enzyme catalysis.The enzyme can reduce the activation energy of the reaction (activation) (△ G) during the reaction, but the reaction rate is accelerated and the reaction time is reduced, but the equilibrium constant is not changed. (2) However, the enzyme is a biological macromolecule (1) Enzyme-catalyzed high efficiency: The catalytic effect of the catalyst can increase the reaction rate by 10 ^ 6 ~ 10 ^ 12 times, which is at least several times higher than that of the conventional catalyst. (2) The enzyme catalyst Highly specificity: including specificity of response, substrate specificity, chirality specificity, geometric specificity, etc., that an enzyme can only act on a certain class or a specific substance. Bond, ester bond, peptide bond and so on can be catalyzed by acid-base hydrolysis, but the hydrolysis of these chemical bonds are different, respectively, the corresponding glycosidase, esterase and peptidase, that is, they were specific (3) enzymatic reaction conditions are mild: enzymatic reaction is generally carried out in aqueous solution of pH = 5 ~ 8, the reaction temperature range is 20 ~ 40 ℃
- Q: Can a catalyst decrease the rate of a chemical reaction? Please give an example if yes.
- no, by defintion the catalyst speeds up the reaction.
- Q: The "one-to-two change" of the catalyst is that the quality and chemical properties of the reactants are constant or the quality and chemical properties of the catalyst are constant?
- The quality and chemical properties of the catalyst are unchanged
- Q: Seems intuitive that it wouldn't, but I dunno the qualitative difference between activation energy & Gibbs free energy. I'M TOO LAZY TO GOOGLE I GOTS STUFF TO DO
- A catalyst can change the activation energy not the Gibbs energy. The Gibbs energy is the energy difference between the initial state and final state. A catalyst cannot change that. Imagine you are driving from school to home. How you drive do not change the height difference between the school and your home. However, a catalyst can change your path which can change the routine you drive from school to home. So if there is a hill in between your school and you home, you have the choice to drive through it or drive around. Here is a picture: upload.wikimedia.org/wikipedia/co... A catalyst can change the height of the barrier, but cannot alter the initial or final state.
- Q: Explain how a catalyst may increase the rate of chemical reaction?
- A catalyst lowers the acitvation energy of a reaction.
- Q: What is the difference between biological enzymes and chemical catalysts?
- The biological enzyme is a class of molecules with moderate molecular weight in the living cells. It is a natural macromolecule catalyst in nature because the enzyme and the reactants are more specific than the reaction of the catalyst with the chemical synthesis of the catalyst (1) High efficiency (2) selectivity good by-product less (3) mild reaction conditions and so on
- Q: What is the reaction of hydrogen peroxide to add manganese dioxide?
- Hydrogen peroxide → water + oxygen (arrows on the manganese dioxide, as a catalyst)
- Q: What is the PTC catalyst in chemistry?
- 1, polyether chain polyethylene glycol: H (OCH2CH2) nOH chain polyethylene glycol dialkyl ether: R (OCH2CH2) nOR2, cyclic crown ethers: 18 crown 6,15 crown 5, Fine and so on. 3, quaternary ammonium salt: commonly used quaternary ammonium salt phase transfer catalyst is benzyl triethyl ammonium chloride (TEBA), tetrabutyl ammonium bromide, tetrabutylammonium chloride, tetrabutylammonium hydrogen sulfate (TBAB) , Trioctylmethylammonium chloride, dodecyltrimethylammonium chloride, tetradecyltrimethylammonium chloride, and the like. 4, tertiary amine: R4N X, pyridine, tributylamine and the like. 5, quaternary ammonium base (its alkaline and sodium hydroxide similar) soluble in water, strong hygroscopicity. 6, quaternary phosphonium
- Q: The catalyst can change the chemical reaction process, why is it wrong?
- The catalyst can change the chemical reaction rate
Send your message to us
Amino Trimethylene Phosphonic Acid Water Treatment Chemical
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords





















