Amino Trimethylene Phosphonic Acid Corrosion Inhibitors
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 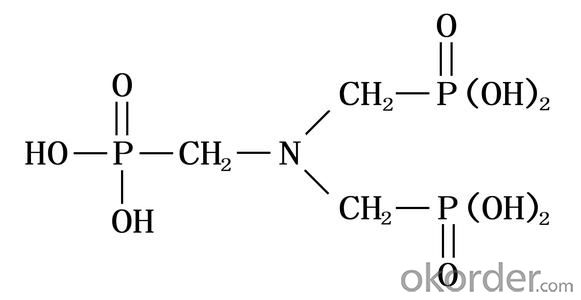
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q: When there is a catalyst in the chemical equation, it is not necessary to match the atoms of the catalyst
- No need, because the catalyst in the chemical reaction before and after the quality of the same
- Q: Does the catalyst participate in chemical reactions?
- The catalyst does not participate in the chemical reaction, it only plays an auxiliary role.
- Q: Chemical production of iodine and magnesium with water as catalyst!
- In the 250mL three bottles were equipped with spherical condenser and constant pressure dropping funnel, in the condensate tube connected to the anhydrous calcium chloride drying tube. The flask was placed with 1.5 g of magnesium chip and a small tablet of iodine, 10 g of bromobenzene and 30 mL of anhydrous ether were mixed in a constant pressure dropping funnel. First 1/4 of the mixture into the flask, a few minutes later see the magnesium surface of the bubble generated, the solution was slightly cloudy, iodine color began to disappear. If no reaction occurs, use a hot water bath. After the start of the reaction, stir, slowly dropping the remaining bromophene ether solution, dropping the rate to keep the solution was slightly boiling state, after adding, in the water bath to continue reflow 0.5h, magnesium tablets full effect.
- Q: Which branch of chemistry or what specialty can study the catalyst
- Inorganic Chemistry: Preparation, Synthesis, Structure and Catalytic Reaction of Inorganic Catalysts and Catalyst Carriers
- Q: What is positive and negative catalyst in chemistry?
- Positive catalyst can speed up the reaction rate, negative catalyst can slow down the reaction rate
- Q: What is the effect of the catalyst in chemistry?
- Changing the rate of reaction can speed up the reaction and slow down the rate of reaction
- Q: What is a catalyst in a chemical reaction?
- A catalyst increases the rate of a reaction by lowering the activation energy. Activation energy is the energy required to make the reactants form the products. If the activation energy is lower the reaction takes place faster and more easily. Also catalysts are not used up during the reaction.
- Q: It is best to tell me what the role of sulfuric acid in these reactions, respectively
- Esterification reaction (dehydration), nitration (dehydration, concentrated nitric acid), carbonation reaction or dehydration reaction (organic matter in sulfuric acid blackening, dehydrating agent), sulfonation reaction (dehydrating agent), ethylene (dehydrating agent).
- Q: Why are catalysts so effective in small amounts?
- By definition, catalysts serve to accelerate certain chemical reactions, by lowering the activation energy required for them to proceed. They are not consumed by the reaction, which is why they are effective in small amounts.
- Q: chemistry subject
- copper nickel zinc common catalysts are solid acids such as the silicas, alumina, and zeolites it depends on the reaction
Send your message to us
Amino Trimethylene Phosphonic Acid Corrosion Inhibitors
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches




















