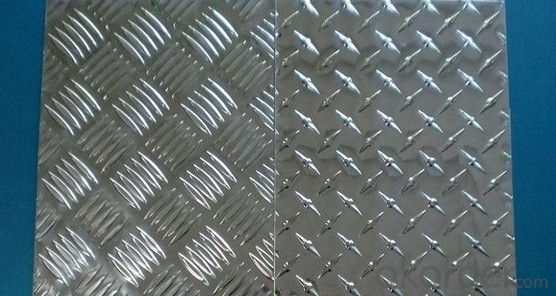
3mm Anti Slip Embossed Aluminum Checker Plate Sheet
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 9000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Specification
Product Description
The brilliant shine of Aluminum Diamond Plate adds sparkle, along with durability. As known as: Aluminum Diamond Plate, Aluminum Tread Plate, Tread Bright, Aluminum Floor Plate, this highly reflective, economical product is widely used in decorative, cosmetic and architectural applications. Having good forming, drilling and welding ability, Aluminum Diamond Plate is easy to fabricate and its raised diamond lug pattern provides good slip resistance.

Specifications for anti slip aluminum sheet:
| Alloy No. | Thickness (mm) | Width (mm) | Length (mm) | Temper | |
| A1050,A1060, A1070,A1100 | 1.0-10 | 20-2200 | 20-8000 | H12,H22,H14,H16,H18, H24,H26,etc | |
| A3003,A3105,A3004 | 1.0-10 | 20-2200 | 20-8000 | H14,H18,H24,etc | |
| A5052,A5005,A5083, A5754 | 1.0-10 | 20-2200 | 20-8000 | H18,H24,H32,H34,H111,H112 ,etc | |
| A6061,A6082,A6063 | 1.0-10 | 20-2200 | 20-8000 | T4,T6,,etc | |
| A8011 | 1.0-10 | 20-2200 | 20-8000 | H12,H22,H14,H16,H18,H24,H26, etc | |
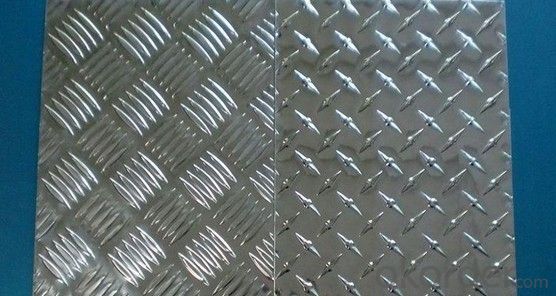
Standards:
ASTM-B209. EN573-1, GB/T3880.1-2006
Quality of material:
totally free from defects like white rust, oil patches, roll marks, edge damage, camber, dents, holes, break lines, scratches and free from coil set
Application :
Mainly used insigns, billboards, building exterior decoration, bus body, high-rise buildings and factories wall decoration, kitchen sink, lamp, fan leaves, with pieces of electronic, chemical equipment, sheet metal processing parts, deep drawing or spinning hollowware, welding parts, heat exchangers, bell surface and disk, plate, kitchenware, decorations, reflective devices, ect.

- Q: What are the distinctions between standard aluminum windows and those that are custom-made?
- <p>Standard aluminum windows are mass-produced and come in set sizes and styles, offering affordability and quick installation. They may not fit perfectly into unique spaces and may lack customization options. Custom-made aluminum windows, on the other hand, are tailored to specific dimensions and design preferences, ensuring a perfect fit and enhanced aesthetics. They often come with more features and options, such as energy efficiency, security, and unique styling, but are typically more expensive and have a longer lead time for production and installation.</p>
- Q: Can aluminum sheet be used for soundproofing?
- Yes, aluminum sheet can be used for soundproofing to some extent. Aluminum is a dense and rigid material, making it effective at blocking sound transmission. When used in conjunction with other soundproofing materials, such as foam or mineral wool, it can further enhance soundproofing capabilities. However, it is important to note that aluminum sheet alone may not provide complete soundproofing, as it may still allow some sound to pass through due to its thin nature. Additionally, proper installation and sealing of any gaps or openings is crucial for maximizing the soundproofing effectiveness of aluminum sheet.
- Q: With a project I'm working on, I need to reinforce a small case made of aluminium. My options are to use regular old screws (probably steel) or Stainless Steel screws (much more costly). Just wondering what corrosion will be like? *It will never ever get wet,* so does that mean corrosion will not occur? (to a certain degree, obviously) And would corrosion, since its dry, still be affected by the type of metal used for the screws? The screws need to be strong, and they also need to be purchase-able at a regular hardware store, as I'm not going to go buying just screws on-line. Thanks for any info you can provide.
- You don't mention just what this project is, but 'regular' screws in the aluminum will not 'rust' - but they may cause galvanic action (a white powdery aluminum oxide may form over time.) Most hardware stores carry aluminum screws, why not just buy and use them if you are concerned? They are usually used for aluminum replacement windows and may be painted white. You can use sandpaper to remove the paint if you want the silver to show.
- Q: I need help with this question for a report i'm doing, it's about recycling aluminium, please could you give me some reasons why to recycle aluminium and eg.saves energy, and how it does eg. saves energy by...Thanks a lot this will help me loads.P.S this is just a report for my science project.
- Aluminum has to be extracted from the earth which is costly and takes a lot of energy. Then the aluminum needs to be smelted out of the rock that it is in, again this takes a lot of energy and creates toxins. Aluminum like all metals are finite, we conserve resources and the land where they will need to be extracted by recycling it.
- Q: The real bumper/ reinforcement bar that is behind the plastic bumpers. Magnet don't stick to it and it don't rust which both types of metal are pretty much like that.
- Aluminum, stainless is too expensive and not ductile enough to be made into bumpers, especially of that complexity. As far as the magnetism, the magnet would only stick to low nickel stainless and its not likely that they would use 308 on a consumer vehicle.
- Q: Trying to find the best aluminum powder I can buy for rocket engines and pyrotechnic (fireworks) uses?
- It really depends on your formula and how the aluminum is being used in the formula. Is it a part of the fuel or is an additive to the fuel for sparks?
- Q: How does the alloy composition affect the mechanical properties of aluminum sheet?
- The mechanical properties of aluminum sheet are significantly influenced by its alloy composition. These properties include tensile strength, yield strength, elongation, hardness, and fatigue strength, among others. To begin with, the overall strength of the aluminum sheet is greatly affected by its alloy composition. Aluminum alloys are commonly mixed with elements like copper, manganese, magnesium, and silicon to enhance their mechanical properties. These alloying elements form solid solutions or precipitates within the aluminum matrix, resulting in a significant increase in material strength. For example, the addition of copper to aluminum forms a solid solution that enhances the sheet's tensile strength and yield strength. Furthermore, the ductility and formability of the aluminum sheet are also impacted by its alloy composition. Some alloying elements, such as magnesium and silicon, can reduce the ductility of aluminum alloys. This reduction in ductility makes the sheet more susceptible to cracking or fracturing when subjected to stress. Conversely, certain alloying elements can improve the formability of aluminum sheet, making it easier to shape or bend without cracking. Moreover, the resistance to corrosion and heat of aluminum sheet is influenced by its alloy composition. For instance, aluminum alloys with a high magnesium content, such as the 5000 series, exhibit excellent corrosion resistance and are commonly used in marine applications. Additionally, specific aluminum alloys are developed for high-temperature applications, with alloying elements like copper and zinc enhancing their heat resistance. Finally, the microstructure of aluminum sheet is affected by its alloy composition, which in turn affects its mechanical properties. Different alloy compositions can result in various microstructural features, including grain size, grain boundaries, and phase distribution. These microstructural characteristics can impact the sheet's strength, hardness, and fatigue resistance. In conclusion, the alloy composition plays a crucial role in determining the mechanical properties of aluminum sheet. Manufacturers can tailor the sheet's properties to meet specific requirements, such as strength, ductility, formability, corrosion resistance, and heat resistance, by carefully selecting the appropriate alloy composition.
- Q: How does aluminum sheet compare to other metals in terms of weight?
- Aluminum sheet is exceptionally lightweight compared to most other metals. It has a lower density, making it a popular choice for applications where weight reduction is a priority.
- Q: This question asks for an explanation of the various types of coatings applied to aluminum sheets that are used outdoors.
- <p>Aluminum sheets used for exterior applications are often coated to enhance their durability, weather resistance, and aesthetic appeal. The different types of coatings include: 1. Anodizing, which creates a protective oxide layer on the aluminum surface. 2. Powder coating, a dry finishing process that provides a uniform and durable finish. 3. PVDF (Polyvinylidene Fluoride) coating, known for its excellent resistance to UV rays and chemicals. 4. Fluorocarbon coatings, which offer superior resistance to weathering and color retention. 5. Electrolytic coating, which involves the application of a thin protective layer through an electrochemical process. Each coating type has specific properties that make it suitable for different exterior applications and environmental conditions.</p>
- Q: Can aluminum sheets be used for storage tanks?
- Yes, aluminum sheets can be used for storage tanks. Aluminum is a lightweight, corrosion-resistant material that is commonly used in various industries, including storage tank manufacturing. It offers durability, excellent thermal conductivity, and is easily formable, making it suitable for storing various liquids and gases.
Send your message to us
3mm Anti Slip Embossed Aluminum Checker Plate Sheet
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 9000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords