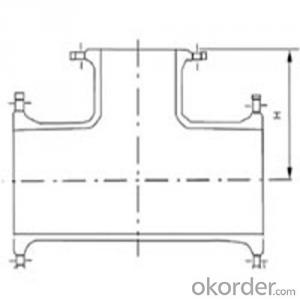
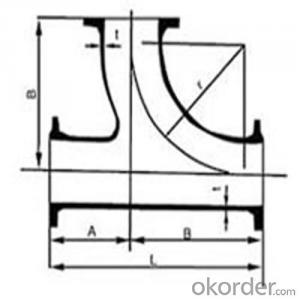
Ductile Iron Bends with Flange
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
Quality Product, Order Online Tracking, Timely Delivery
OKorder Financial Service
Credit Rating, Credit Services, Credit Purchasing
You Might Also Like
Specifications
ISO2531/EN545 Ductile iron Double flange bend pipe fittings with epoxy coating
1.standard:ISO2531/EN545/EN598
ISO2531/EN545 Ductile iron Double flange bend pipe fittings with epoxy coating
This is SHANXI ASCENT INDUSTRIAL CO.,LTD,
2. material : GGG500-7 or other Ductile iron .
3. coating : Fusion bonded epoxy coating ; cement lining inside and zinc primer and bitumen painting outside ; bitumen painting inside and outside ; red anti-rust coating .
4. package : wooden cases with plastic layer , wooden pallets with plastic layer , steel crates .
5. accessories such as gaskets , bolts and nuts available upon request .
- Q:Don‘t want to iron sheets!
- then dry them and right out of the drier fold them don't wait if they get too cold it will hold wrinkles! or get them dry cleaned!
- Q:So I am trying to increase my iron levels as I have to give blood soon. Well I picked up an iron supplement and I took one. Well I just realized that it has 65 mg of iron in one pill. Why would they sell these if toxicity is over 50mg. Am I gonna be okay?
- It is great that you are giving blood. However, unless you are suffering from iron deficiency anemia, no amount of iron is going to increase your hemoglobin count. You could take iron after the event, to speed up recovery, but most people do not have a problem in getting back to a normal level. 65mg of elemental iron ( usually in salt of iron about 300mg ) is the treatment dose for simple anemia. Toxic levels for an adult male start at about 700mg of elemental iron daily. If your hemoglobin level is normal, you need no more than 20mg daily for good health. Plenty of foods contain iron including green vegetables and liver
- Q:I have build-up on my flat iron from products that were in my hair. How can i clean it off without scratching up the metal?
- I think we did this experiment at school, i think it was Distilled water - no rust Tap water - very rusty Chemical - Slightly rusty pretty sure that's right, if not, i'm sorry. :)
- Q:You have a spool of wire, a glass rod an iron rod and an aluminium rod. Which rod should you use to make an electormagnet to pick up steel objects? Explain
- It is fairly easy to build an electromagnet. All you need to do is wrap some insulated copper wire around an iron core. If you attach a battery to the wire, an electric current will begin to flow and the iron core will become magnetized. When the battery is disconnected, the iron core will lose its magnetism.
- Q:Im looking at buying some new irons, i have a 16 handicap and i wanted some feedback on clevelands gold and red irons and also the hibore ironsat 16 handicap will it be a mistake to go with the red irons?
- probably a wiring glitch, I had one of these incidents in my car, and I had to strip the whole system, and fix it. I suggest to get a new alarm and have it installed by a pro mechanic ($300)
- Q:1. Can they use rusted iron (since it is in the water)2. How does the iron keep from sinking since they live at the surface
- Phytoplankton require a variety of essential nutrients, including iron, to fix carbon dioxide and fuel ocean food webs, but the oxidized form of iron, Fe(III), that prevails in the ocean is only sparingly soluble in oxygenated seawater. The solubility of iron is enhanced through chelation with organic ligands, and nearly all of the dissolved iron in seawater is bound to natural ligands. More details in link.
- Q:why in acid solution gold is not corroded??why in acid solution iron is hardly corroded??
- Gold is a non-corrosive element (no free electrons in relation to the number of protons in the nucleus), in that it will not combine with oxygen to produce gold-oxide (corrosion), and it is one of the best conductors of electricity of all the elements. Which is why gold plating is selected for use in electronic circuits where corrosion can be a problem. Iron in an acid solution hardly corrodes because the acid solution may contain very little oxygen. Put the iron in an oxygen-rich environment and the corrosion will increase dramatically in the form of iron-oxide (rust). This is why pure iron is not a good choice where corrosion could be a problem, unless measures are taken to prevent or minimize the corrosive properties of the surrounding environment. As an example, when ductile iron is used for water pipes, it is necessary to set up corrosion-control electrical systems and to isolate the ductile iron pipes where they connect to other pipes that could induce an electrolytic flow, and they are coated with mortar to prevent contact with the soil. Monitoring stations are set up to test the pipe and monitor the amount of electrolysis in the pipe.
- Q:Hi,Since ferritin stores iron as Fe 2+ (which is toxic to cells), how does it release the iron without causing cell death? Thank you!
- power surge from charging system could have blown out your brake light
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Ductile Iron Bends with Flange
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
Quality Product, Order Online Tracking, Timely Delivery
OKorder Financial Service
Credit Rating, Credit Services, Credit Purchasing
Similar products
New products
Hot products
Related keywords