Good Quality of Calcium Carbide with Competitive Price
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 22.5
- Supply Capability:
- 2400 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
1. Structure of Calcium Carbide Description:
CAS NO.:75-20-7
MF:CaC2
Grade Standard: Industrial Grade
Standard:GB10665-2004
HS Code:28491000
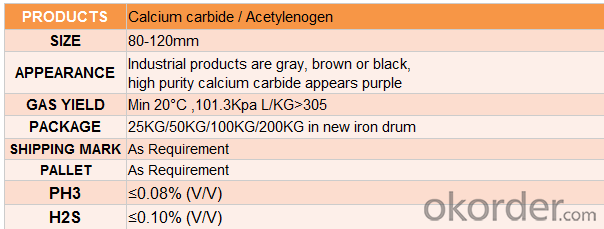
Purity: Gas Yield:295L/KG MIN
Packing&Delievery:50kg~100kg/IRON DRUM
All year Delievery
2. Main Features of Calcium Carbide:
This product is lumpy substance, its surface is a little deep gray, has slight nasty smell. It can produce acetylene gas when met water, it can burn when meets fire. The acetylene gas mix with air will form explosive gas (explosive range of acetylene gas in the air is 2.3% - 81%).
3.Calcium Carbide Images



4.Calcium Carbide Specification
5.FAQ
1)How many tons does your factory can supply each moth?
30000tons/month
2)How to quarantee the quality of the products?
you can arrange SGS&BV or other quality inspection.
3)How many days you need to pepare the cargo after we made the order?
within 30 days.
- Q:What is the difference between chemical monomer and elemental
- And the element is composed of the same element of pure matter. An element is called a free state of an element in the presence of an element. In general, the nature of the element is closely related to the nature of its element. For example, the metal properties of many metals are obvious, then their elemental reducibility is very strong. Different elements of the element, the nature of the differences reflected in the structure of the most prominent. In contrast to elemental matter, a substance consisting of multiple elements is called a compound
- Q:Is sulfur simple?
- Say that sulfur is simple
- Q:What is the elemental element
- Elemental iron, Fe gas Elemental O2 These are the same kind of elements composed of the same kind of molecules / ions / atoms
- Q:What are the two kinds of prime
- Metal or non-metallic. Either a single molecule or a multi-molecule. Depending on the different points.
- Q:What are the black solid elements in junior high school chemistry?
- C (charcoal, graphite)
- Q:What is a compound?
- Compounds are sub-states called molecular compounds, such as water, sugar and so on. Compounds are ionically bonded as ionic compounds, such as salt, mirabilite and the like. The compound may be represented by a chemical formula consisting of the symbols of the elements contained in the compound. For example, binary compounds consisting of two elements, in writing their chemical formula, as the name of its English name, metal elements written in the former, and less metal followed. Such as salt (sodium chloride) chemical formula for the NaCl. When the number of atoms in the different elements of the compound is not equal, the ratio can be written under the symbol. If the chemical formula of sucrose is C12H33O11, it means that the sugar consists of twelve carbon atoms, twenty-two hydrogen atoms, and eleven oxygen atoms.
- Q:Diamond graphite c60 are simple elements do?
- Diamond, graphite, C60 are composed of carbon elements of the elemental, diamond, graphite is composed of carbon atoms, C60 is composed of molecules that C60 molecules, diamond and graphite due to the different arrangement of carbon atoms so there is a great physical properties Differences, the diamond is the natural existence of the most hardest material, graphite can be conductive to do the electrode, activated carbon and charcoal can adsorb toxic gases and pigments, carbon simple chemical properties similar to both flammability and reductive, combustion to produce carbon dioxide.
- Q:Diamond, graphite is a simple element?
- Is the elemental, but the molecular structure is not the same. There is a chemical bond in the same element.
- Q:What are the different elements of the same element?
- The same kind of elements are pure substances, such as: oxygen, hydrogen. Different elements can also form a pure substance, such as: carbon monoxide, carbon dioxide (water is a mixture of) It depends on the situation, the same element can be composed of different elements, : Oxygen and ozone, one is two oxygen atoms, one is three oxygen atoms.
- Q:Does the mixture have a simple substance? What is it?
- Of course there are myself! Such as red phosphorus and white phosphorus mixture, white tin and gray tin mixture, oxygen and ozone mixture, etc., anyway, there are a lot of it! Usually pay attention to accumulation. I hope that my answer is helpful to you and wish you good results!
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Good Quality of Calcium Carbide with Competitive Price
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 22.5
- Supply Capability:
- 2400 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Hot Searches




























