Dental Floss Pick Bulk 50 pcs OEM Wheat Straw ECO Friendly Dental Floss
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 200 bag
- Supply Capability:
- 100000 bag/month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Specification
Dental Floss Pick Bulk 50 pcs OEM Wheat Straw ECO Friendly Dental Floss
| Brand | OEM/Ok Floss |
| Floss material | UHMWPE+ wheat Straw |
| Floss length | 7.4*2.2cm/OEM |
| Wax | Import germany wax/american wax/bee wax/candelilla wax/customized wax |
| Flavor | Mint / green tea / lemon / peach / fruit / aloe / gin |
| Color | Floss original color |
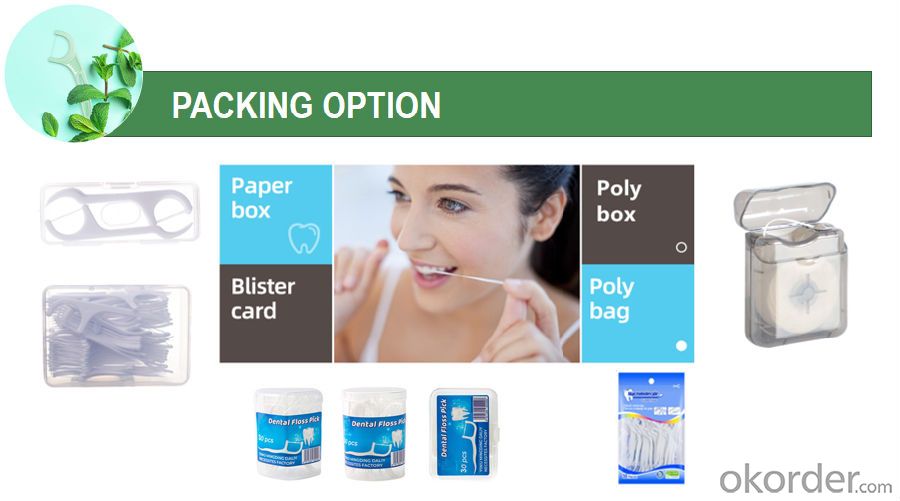
| Floss original color | Individual bulk PP bag/individual paper box/as customized |
| Applications | Family,Hotel,Travel,Supermarket |
| Delivery | By sea,By air,By express. |
| Lead time | 20-30days |
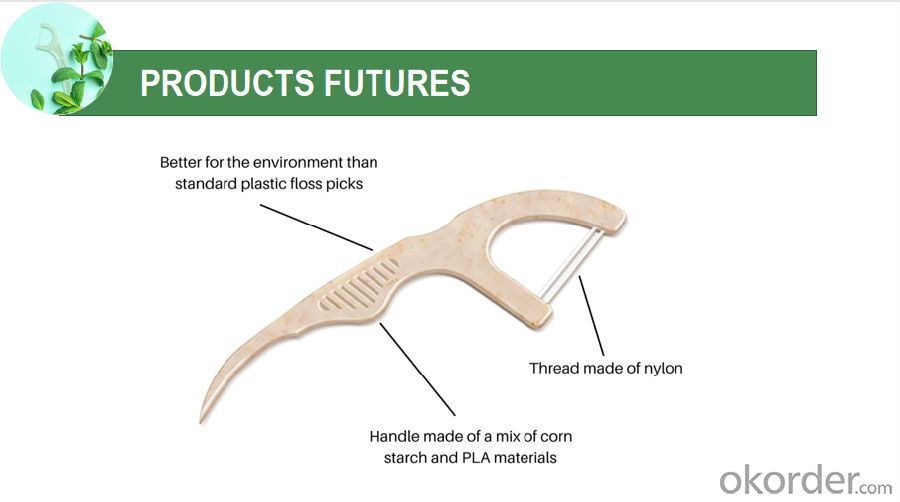
Soft tape to ace the tight space
No break, no shred floss
Helps remove plaque between teeth
Helps prevent gingivitis between teeth
Unique handle design, makes it easy and comfortable to floss. Removes trapped food debris.
Extra bristled pick for hard to get bits,even gets behind hard-to-reach molars.
Gently slides smooth between tight teeth. Freshen breath with flavor.
Recommends you use OK flosser after every meal and snack to have a fresher, cleaner mouth. For a completely daily clean, use with a OK flosser to easily reach back teeth.

 Why we need use dental floss ?
Why we need use dental floss ?
Because it can reduce your chances of 60% of
adjacent dental caries and 70% of periodontal
disease.
It allows you to have a healthy tooth of your own at
the age of 80.
Flossing your teeth in the right way will remove
food particles and growing plaque from spots
where your toothbrush can’t reach, generally
between your teeth and under the gumline.






Suggested use
Directions for flossing: While holding the handle, gently guide the floss between your teeth using a zig-zag motion. Move floss away from the gumline in a gentle back and forth, up and down motion alongside each tooth to remove food particles and plaque. Rinse floss as needed and repeat for each tooth.
Warnings
Adult supervision recommended under age 10.
FAQ
Q: Can you accept OEM make?
A: Sure.We can make your customized brand and design blister card, inner box and master carton, and also pack as per your request.
2. Q: Where can I put my logo?
A: We can print your logo on the sticker, blister card, inner box and master carton.
3. Q: What are your MOQ?
A: Blister card packing: 10000 pcs/item.
Bulk packing: 5000 pcs/item.
4. Q: How can I get Samples?
A: Samples are free, while the shipping cost to be collected.
5. Q: What are your terms of payment?
A: 1) T/T(30% in advance, the balance by the copy of B/L).
2)For small order less than USD 3000, in order to save bank commision for both of us, we need 100% in advance.
6. Q: How long is the delivery time?
A: For the MOQ, the leading time is about 20-30 days.
- Q: How do medical plastics contribute to the development of point-of-care testing?
- Medical plastics play a crucial role in the development of point-of-care testing by providing essential components such as microfluidic chips, biosensors, and disposable devices. These plastics are lightweight, durable, and cost-effective, allowing for portable and convenient point-of-care testing devices to be developed. Additionally, medical plastics can be easily molded into various shapes and sizes, enabling the miniaturization and integration of multiple functions into a single device. This contributes to the advancement of point-of-care testing by improving accuracy, speed, and accessibility of diagnostic tests, ultimately leading to better patient outcomes and reduced healthcare costs.
- Q: How is medical plastic used in telemedicine devices?
- Medical plastic is used in telemedicine devices for various purposes. It is commonly used to manufacture the casings and components of telemedicine devices, ensuring their durability and reliability. Medical plastic is also utilized for creating the necessary barriers and protective covers for telemedicine equipment, ensuring the safety and hygiene of both patients and healthcare providers. Additionally, medical plastic is often used to create disposable components such as tubing, connectors, and sensors, which are crucial for accurate data transmission and monitoring in telemedicine applications. Overall, medical plastic plays a crucial role in the design, functionality, and safety of telemedicine devices.
- Q: How do medical plastics handle exposure to different chemicals?
- Medical plastics are designed to be chemically resistant and can handle exposure to a wide range of chemicals commonly used in healthcare settings. They are typically made from materials such as polypropylene, polyethylene, or polycarbonate that have high chemical resistance. These plastics can withstand exposure to disinfectants, cleaning agents, and many other chemicals without degrading or leaching harmful substances into the surrounding environment. Additionally, medical plastics are often tested and certified to ensure their compatibility with various chemical substances, making them suitable for a variety of medical applications.
- Q: How does medical plastic contribute to the development of biosensors?
- Medical plastic plays a crucial role in the development of biosensors as it provides a biocompatible and versatile material for sensor components. The properties of medical plastic, such as transparency, flexibility, and ease of fabrication, allow for the creation of highly sensitive and miniaturized biosensors. Additionally, medical plastic can be modified to enhance its compatibility with biological samples, enabling closer integration of the biosensor with the human body. Overall, medical plastic enables the advancement of biosensors by providing a suitable platform for the detection and monitoring of various medical conditions.
- Q: Can we use black plastic bags for medical waste?
- Plastic bags for medical waste are not related to the color of plastic bags. In other words, plastic bags of any color can be used to hold medical waste.
- Q: Classification and marking of plastic products
- 04 - LDPE (low density polyethylene), plastic film, plastic film and so on are all of this material. Heat resistance is not strong, usually qualified PE cling film will appear in the temperature of more than 110 degrees of hot melt phenomenon, will leave some of the body can not be broken down the plastic preparations. And wrap the food with plastic wrap to heat it. The grease in the food easily dissolves the harmful substances in the preservative film. Therefore, the food into the microwave oven, it is necessary to remove the wrapped plastic wrap.
- Q: How is medical plastic used in sleep medicine?
- Medical plastic is used in sleep medicine for various purposes. It is commonly used to manufacture devices such as continuous positive airway pressure (CPAP) masks, which are used to treat sleep apnea by delivering a constant flow of air to keep the airway open during sleep. Medical plastic is also used in the production of dental devices like mandibular advancement devices (MADs), which are worn during sleep to treat snoring and mild to moderate sleep apnea. Additionally, medical plastic is used in the construction of various sleep monitoring devices, such as pulse oximeters and sleep sensors, which help in diagnosing and monitoring sleep disorders. Overall, medical plastic plays a crucial role in ensuring the comfort, safety, and effectiveness of sleep medicine devices and aids in improving sleep quality and overall well-being.
- Q: What is non PVC soft bag infusion?
- Is the use of non PVC soft bag composite film to replace the glass bottle for infusion packaging, the soft bag is made of three layer coextrusion film, without the use of adhesives, cleaning membrane, soft bag molding was completed in 100 grade clean workshop, pyrogen free, particles.
- Q: Can medical plastics be reused?
- No, medical plastics cannot be reused due to the risk of contamination and potential compromise of sterile conditions.
- Q: Can medical plastic be safely used in geriatric applications?
- Yes, medical plastic can safely be used in geriatric applications. Medical plastics are specially designed to meet the unique needs of the healthcare industry, including geriatric care. They are durable, non-toxic, and resistant to bacteria, making them suitable for a wide range of medical devices and equipment used in geriatric care settings. Additionally, medical plastics can be easily cleaned and sterilized, ensuring the safety and well-being of elderly patients.
Send your message to us
Dental Floss Pick Bulk 50 pcs OEM Wheat Straw ECO Friendly Dental Floss
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 200 bag
- Supply Capability:
- 100000 bag/month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords