1060 Aluminum Coils Wholesale - Aluminium Profile 180T Best Price and Good Quality
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 15 m.t.
- Supply Capability:
- 2000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Specification
Structure of Aluminium Profile 180T Best Price and Good Quality Description:
Coated aluminum coil/sheet are of a wide range of colors, which gives wonderful appearance no matter in residential and commercial constructions of great exhibition centers.
The coated aluminum coil/sheet have been widely used in the fields of construction and decoration( garage doors, ceiling etc.), electronic appliances, lighting decoration, air-condition air pipes, sandwich panels and drainages etc.
Main Features of the Aluminium Profile 180T Best Price and Good Quality:
1) High flexibility
2) Impact resistance
3) Excellent weather-proof durability
4) Anti-ultraviolet
5) High erosion resist
Images of the Aluminium Profile 180T Best Price and Good Quality:
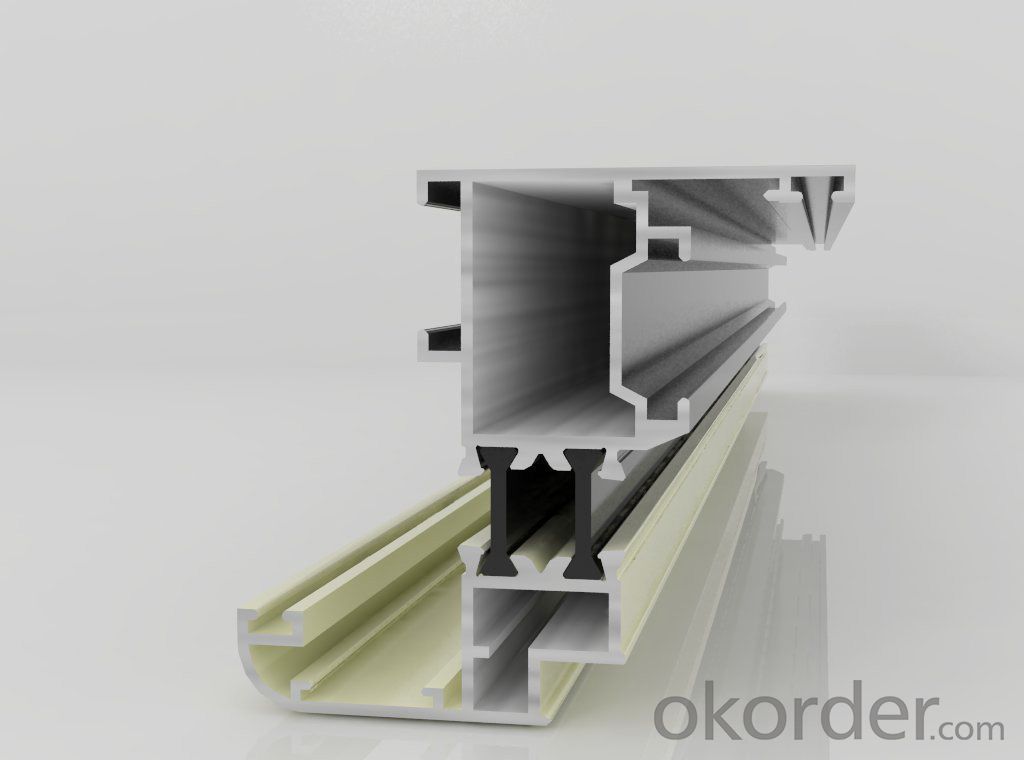
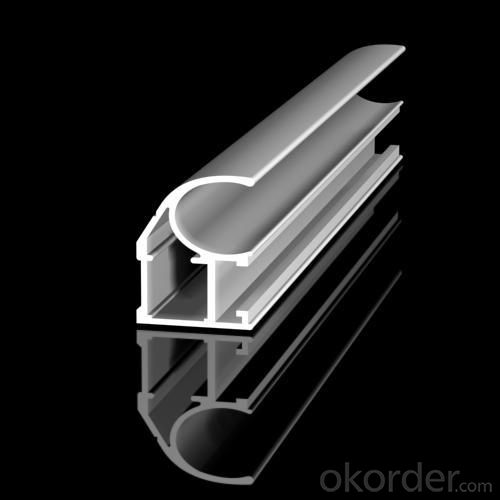

Aluminium Profile 180T Best Price and Good Quality Specification:
Alloy | A1100,A3003,A1050,A8011 etc |
Temper | H16,H18,H24 |
Thickness | From 0.024mm to 1.2mm |
Width | Standard width:1240mm |
Special width:1300mm,1520mm,1570mm,1595mm | |
Diameter | Standard dia:1200mm |
Interior dia:150mm,405mm,505mm | |
Weight | 2.5 T/coil,3.0 T/coil |
Coating | PE, PVDF, AC |
Surface | Embossed, mill finish, coated |
Color | AS to code RAL |
Gloss | 10-90%(EN ISO-2813:1994) |
Coating Thickness | PE: more than 18 micron |
PVDF: more than 25 micron | |
Coating Hardness (pencil resistance) | More than 2h |
Coating adhesion | 5J(EN ISO-2409:1994) |
Impact Resistance | No peeling or cracking(50 kg/cm,ASTMD-2794:1993) |
Flexibility (T-bend) | 2T |
MEK resistance | More than 100 |
FAQ:
a.What is monthly capacity
---CNBM is one stated own company and our monthly capacity is about 2000tons.
b. Now which countries do you export your goods?
---Now we export to South East Asia,Africa, North America,South America ect.
- Q: How are aluminum coils tested for mechanical properties?
- Aluminum coils are tested for mechanical properties through a series of standardized tests that evaluate their strength, ductility, and other relevant properties. Some common methods used for testing aluminum coils include tensile testing, hardness testing, and bend testing. Tensile testing is one of the most widely used methods to assess the mechanical properties of aluminum coils. This test involves applying a pulling force to a small section of the coil until it breaks. The force and elongation are measured throughout the test, and these values help determine the coil's ultimate tensile strength, yield strength, and elongation at break. Hardness testing is another important evaluation method that measures the resistance of the aluminum coil to indentation or scratching. It gives an indication of the coil's ability to withstand external forces. Different hardness testing methods, such as Brinell, Rockwell, and Vickers, can be used depending on the specific requirements. Bend testing is performed to assess the ductility and formability of the aluminum coil. This test involves bending the coil to a specific angle or radius and examining it for any signs of cracking, wrinkling, or other defects. It helps determine the coil's ability to undergo forming processes without failure. In addition to these standard tests, other mechanical properties such as fatigue resistance, impact resistance, and fracture toughness can be evaluated through specialized testing methods. These tests provide valuable information about the aluminum coil's performance and help ensure that it meets the required specifications and standards. Overall, a combination of tests is employed to comprehensively assess the mechanical properties of aluminum coils, ensuring they have the necessary strength, ductility, and other properties needed for their intended applications.
- Q: also, can aluminum become oxidized when secured down with metal nuts and bolts vice stainless steel nuts and bolts?
- Aluminum is very reactive to caustic substances, as well as acids. One of the perils of running nitrous oxide injection on a motor with aluminum heads or pistons is that you generate trace amounts of nitric acid which will corrode these parts over time. As mentioned here by other members, aluminum oxide is a powdery white residue. In the presence of water for a long period of time, or with the reactions mentioned above, aluminum will oxidize. If it's wheels you are talking about, the best solution is to keep them clean, dry, and free of brake dust. A lot of wheels though come with a coating to protect the aluminum.
- Q: Can aluminum coils be used for food processing conveyor systems?
- Yes, aluminum coils can be used for food processing conveyor systems. Aluminum is a durable and lightweight material that is resistant to corrosion, making it suitable for food processing environments. Additionally, aluminum is non-toxic and does not react with food, ensuring the safety and quality of the processed products.
- Q: What will be the pros and cons of using each of these materials?One thing to keep in mind is that aluminum alloys are good conductors of electricity.
- get fiber glass,they will last a long time
- Q: Can aluminum coils be used in cryogenic applications?
- Yes, aluminum coils can be used in cryogenic applications. Aluminum has excellent thermal conductivity and low thermal expansion properties, making it suitable for use in extremely low temperature environments. Additionally, aluminum is resistant to corrosion, which is important in cryogenic applications where moisture and other contaminants can cause damage to the coils.
- Q: which metal is very common today and which one will be most common in future. steel or aluminum
- aluminum will be most commonly use in the future because it is lightweight. But metal is still the most commonly used today.
- Q: I would like to know why the ionic substance aluminium oxide doesn't dissolve in water.
- Aluminium gives away 3 electrons, and two aluminium atoms are combined with 3 oxygen atoms, the charge is just to great for it to gracefully dissolve.
- Q: Are aluminum coils suitable for roofing?
- Absolutely, roofing can indeed make use of aluminum coils. Aluminum, a material highly durable and lightweight, has found extensive application in the construction sector for roofing. It presents numerous advantages, including resistance against rust, corrosion, and fire, rendering it an optimal choice for regions enduring severe weather or heightened humidity. Furthermore, aluminum coils boast exceptional thermal conductivity, facilitating the reflection of sunlight and consequent reduction in energy consumption, thereby resulting in decreased cooling expenses. Moreover, the installation and maintenance of aluminum roofing are easily accomplished, ensuring enduring safeguarding for residential, commercial, and industrial structures.
- Q: Can aluminum coils be used in high-radiation environments?
- Yes, aluminum coils can be used in high-radiation environments. Aluminum has good radiation resistance and can withstand high levels of radiation without significant degradation or damage. It is commonly used in applications such as nuclear power plants and aerospace industries, where radiation exposure is a concern.
- Q: Can aluminum coils be used in vacuum applications?
- Yes, aluminum coils can be used in vacuum applications. Aluminum is a common choice for vacuum systems due to its low outgassing properties, high thermal conductivity, and resistance to corrosion. It is suitable for various vacuum applications such as cooling, heating, and heat transfer.
Send your message to us
1060 Aluminum Coils Wholesale - Aluminium Profile 180T Best Price and Good Quality
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 15 m.t.
- Supply Capability:
- 2000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords