Factory Hot Sell Amino Trimethylene Phosphonic Acid
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 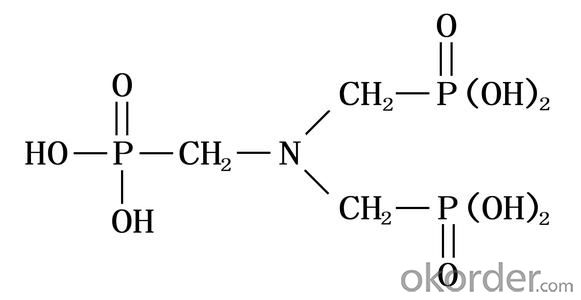
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q: Have you Read it ? If SoCan You Think Of Any Good Group Discussion Questions ?
- no but aOMG i laurie halse anderson! OMG I LOVED HER BOOK FEVER 1793 wooooooooooo that book was aweomse. you should read it
- Q: what is the role of a catalyst in a chemical reaction?
- It increases the rate of reaction by lowering the requirement of energy needed to carry out the chemical reaction. Hope that helped.
- Q: Cl + O3 ---> ClO + O2O + ClO ---> Cl + O2= O + O3 ----> 2O2What is the catalyst? The intermediate?How do you know which is which? If the rate law is rate=k [O3] [Cl]determine:a) the overall order.b) unit for k.c) the rate determining step, justify your answer.
- Cl is the catalyst. ClO the intermediate. The catalyst is the component which does not change in overall reaction. He forms some intermediate component(s) with the reactants. In the later reaction steps the intermediate(s) react forming the catalyst in its original state. (a) The overall order is the sum of the orders with respect to the components: n = 1 +1 = 2 (b) the unit of the rate of reaction is r [=] mol/ (Ls) (more general mol per unit time and volume) compare dimensions mol / (Ls) [=] k · mo/L · mol/L =k [=] L/(s mol) (more general unit volume per unit time and mole) (c) First reaction For elementary reaction steps the order of the reaction rate with respect to a reactant is equal to stoichiometric coefficient. Hence the rate of first reaction is: r? = k?·[Cl]·[O?] Overall rate is given by the rate determining step, while other reaction steps are in equilibrium: r = r? = k?·[Cl]·[O?] If second reaction is the rate determine step r? = k?·[O]·[ClO] while reaction 1 is at equilibrium K? = ( [ClO]·[O?] ) / ( [Cl]·[O?] ) =[ClO] = K?·( [Cl]·[O?] ) / [O?] the overall rate would be: r = r? = k?·[O]·[ClO] = K?·k?·[O]·[Cl]·[O?] / [O?] = k·[O]·[Cl]·[O?] / [O?] That doesn't match the observed rate law
- Q: In the catalyst and light conditions to break down the water to get the chemical equation of hydrogen
- 2H2O = 2H2 ↑ + O2 ↑
- Q: To write a 1500 words of small papers, so please help you busy
- Want to write a good article do not reach out.
- Q: What suitable catalysts can I use for the reaction between Hydrochloric acid and zinc metal?
- catalysts are substances that alter the rate of a reaction.a suitable catalyst would be finely divided platinum.
- Q: Why extract the genome, the digestion is always not cut
- The process of metabolism in the body contains many complex and regular material metabolism and energy changes.Green plants and certain bacteria use solar energy, water, CO2 and inorganic salts and other simple substances, after a series of changes, the synthesis of complex sugar, Fat, protein and other macromolecules, and animals and the use of these plants in the material, and through the complex decomposition and synthesis, the reaction into their own part of the need to grow, breeding, etc. In the laboratory, complex The synthesis and decomposition of organic matter must be carried out under severe conditions such as high temperature, high pressure, strong acid and alkali, such as starch and protein hydrolysis, and some reactions are difficult to carry out in vitro, such as protein synthesis, but in biological conditions Mild (370C or so, near neutral pH), these reactions can be carried out smoothly and quickly.For example, animals eat meat in the digestive tract only a few hours will be completely digested and decomposed; bacteria in the appropriate conditions, Twenty minutes to proliferate generation, in this short twenty minutes, the synthesis of new cells within the need for all the complex substances, etc., what is the reason? This makes the chemical reaction in the body easier And the root cause of rapid progress is the prevalence of a catalytic role in the body of the protein - enzyme.
- Q: What kind of compounds or elements can be used as catalysts in high school chemistry? What is the catalyst for what?
- High school is the most common is manganese dioxide, and potassium permanganate heating oxygen, hydrogen peroxide decomposition are used, which is inferred in the problem there is a lot of
- Q: Why would the Eact decrease if a catalyst is added?
- Catalysts work by providing an (alternative) mechanism involving a different transition state and lower activation energy. The effect of this is that more molecular collisions have the energy needed to reach the transition state. Hence, catalysts can perform reactions that, albeit thermodynamically feasible, would not run without the presence of a catalyst, or perform them much faster, more specific, or at lower temperatures. This can be observed on a Boltzmann distribution and energy profile diagram. This means that catalysts reduce the amount of energy needed to start a chemical reaction.
- Q: How does the catalyst generally add to the organic chemical reaction?
- In the case of heterogeneous catalysis, the specific surface of the catalyst, in addition to the activity of the catalyst, is an important factor in determining the reaction rate, which means that the specific surface for catalysis is large and the reaction is as fast as the whole. So the overall principle in understanding the activity of the premise of the catalyst, would like to quickly add a little more.
Send your message to us
Factory Hot Sell Amino Trimethylene Phosphonic Acid
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches




















