Aluminum Panel Ceiling Sheet From China Wholesaler
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 100000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Item specifice
1.Structure of Aluminum Panel Ceiling Sheet From China Wholesaler
Aluminum Sheets are strengthened and cut from raw materials with different alloys, such as AA5005, AA5052, etc. They are easy for processing in different shapes, good in intensity and can be quickly installed. Aluminium Sheets for Energy Saving Curtain Walls are good in energy saving, weather resistance, fire resistance, easy for maintenance and with many colors.
Aluminium Sheets for Energy Saving Curtain Walls are widely used in construction of metal walls, metal ceilings, car decoration, advertizing panels, etc.
2.Main Features of Aluminum Panel Ceiling Sheet From China Wholesaler
•High intensity
•Easy to be processed and shaped
•Weather resistance
•Anti-pollution & environment protection
3. Aluminum Panel Ceiling Sheet From China Wholesaler Images
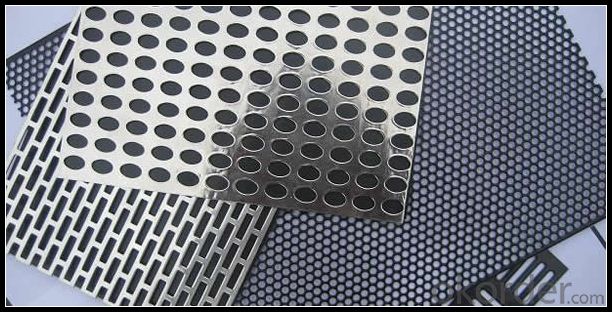


4.Specification of Aluminum Panel Ceiling Sheet From China Wholesaler
Alloy Number | AA5XXX |
Temper | H12, H14, H16, H18, H22, H24, H26, H32, HO, F |
Thickness | 0.1mm – 500mm |
Width | 10mm- 2200mm |
Standard | GB/T3880-2006, ASTM, ISO, EU standard |
5.FAQ
A.What about inspections to guarantee quality?
For each order for Aluminum Sheets with Mill Finished Surface AA5XXX, we will arrange strict inspection for raw materials, inspection during production and inspection for finished goods.
With requirement of customers, we also can arrange the third party inspection.
B.What about delivery?
We will put order for Aluminum Sheets with Mill Finished Surface AA5XXX in production schedule after order gets confirmed against copy of TT or L/C. Normally it takes about one month for production. Exact shipment schedule is different based on different sizes and quantity.
C.What is the MOQ?
5 tons for each size.
D. Where have you exported aluminium sheets?
We have exported aluminum sheets to many countries. Main markets include South East Asia, Middle East, North America, South America, etc.
- Q:Are the aluminum sheets recyclable?
- Yes, aluminum sheets are highly recyclable. Aluminum is one of the most recyclable materials on the planet, and it can be recycled indefinitely without losing its quality. Recycling aluminum sheets requires less energy compared to producing new aluminum from raw materials, which has significant environmental benefits. The recycling process involves melting down the aluminum sheets to extract the metal, which can then be used to produce new aluminum products. Recycling aluminum sheets not only conserves valuable resources but also helps reduce greenhouse gas emissions and landfill waste. Therefore, it is highly recommended to recycle aluminum sheets whenever possible.
- Q:What is the typical machinability of aluminum sheets?
- Aluminum sheets are widely acknowledged for their excellent machinability. This is due to their relatively low density and softness, which make them easier to machine compared to other metals. Furthermore, aluminum exhibits good thermal conductivity, enabling efficient heat removal during machining. Its low melting point also reduces the likelihood of overheating. Moreover, aluminum demonstrates favorable chip formation characteristics, resulting in effortless chip removal and decreased tool wear. However, the machinability of aluminum may differ depending on factors like alloy composition, tempering, and surface finish. Nevertheless, aluminum sheets are generally considered to be highly machinable.
- Q:What are the different methods of surface protection for aluminum sheet?
- Some of the different methods of surface protection for aluminum sheet include anodizing, powder coating, painting, and laminating. Anodizing involves creating a protective oxide layer on the surface of the aluminum, which provides corrosion resistance and enhances its appearance. Powder coating involves applying a dry powder coating to the aluminum sheet and then curing it through heat, creating a durable and protective finish. Painting is another method where a liquid paint is applied to the surface of the aluminum sheet, providing both protection and aesthetic appeal. Laminating involves bonding a protective film or sheet onto the surface of the aluminum, offering resistance against scratches, abrasion, and chemical damage. These methods help to preserve the quality and longevity of the aluminum sheet in various applications.
- Q:According to the reactivity of metals, aluminum chloride (AlCl3) will not react with copper (Cu). But I am almost sure that the copper nail I put in the aluminum chloride solution became shiny and lost its copper lust. Why did this reaction happen?
- it particularly is authentic that many deodorants, quite those categorized anti-perspirants, incorporate aluminum chloride as an lively ingredient. Aluminum chloride does decrease sweat production via the sweat glands that are placed interior the floor and are quite ample under the hands. For some people who're heavy sweaters this drying consequence is amazingly significant. despite the fact that those deodorants decrease sweat production they do no longer block the sweat glands. we are no longer attentive to any at as quickly as risky outcomes on maximum persons from making use of a deodorant containing aluminum chloride. In our very close society at present, physique hygiene and prevention of physique smell are significant. in case you do no longer use a deodorant you are able to discover your persons the two complaining or keeping off you. while you're worried with reference to the drying consequence seem for a deodorant that would not incorporate aluminum chloride.
- Q:Are aluminum sheets suitable for use in HVAC (heating, ventilation, and air conditioning) systems?
- Aluminum sheets are indeed a fitting choice for HVAC systems. The popularity of aluminum in HVAC systems stems from its numerous advantageous properties. To begin, aluminum is both lightweight and robust, making it effortless to handle and install in HVAC systems. Its low density also enables cost-effective transportation and reduces the overall weight of the HVAC units. Furthermore, aluminum possesses exceptional resistance to corrosion. Given that HVAC systems often encounter moisture and condensation, particularly in air conditioning units, aluminum's corrosion resistance ensures its durability and longevity in such environments. Moreover, aluminum sheets exhibit commendable thermal conductivity, facilitating efficient heat transfer throughout the HVAC system. This property holds particular significance in heating and cooling applications, as it aids in effectively distributing the desired temperature. In addition, aluminum is a non-toxic material, rendering it safe for use in HVAC systems that circulate air within buildings. It does not emit any harmful particles or gases, thereby ensuring the quality of indoor air. Lastly, aluminum is highly recyclable, contributing to its environmental friendliness. The ability to recycle aluminum sheets reduces the carbon footprint associated with HVAC systems and aligns with sustainability objectives. Taking all these advantages into account, aluminum sheets undeniably prove suitable for use in HVAC systems. They offer an optimal combination of strength, corrosion resistance, thermal conductivity, safety, and environmental friendliness.
- Q:How does aluminum sheet perform in extreme temperatures?
- Due to its unique properties, aluminum sheet exhibits exceptional performance in extreme temperatures. One of its key advantages is its remarkable thermal conductivity, enabling it to efficiently transfer heat. This characteristic allows aluminum sheet to quickly adapt to extreme temperatures, preventing the occurrence of warping or cracking that may be experienced with other materials. Moreover, aluminum possesses a high melting point of approximately 660 degrees Celsius, rendering it highly suitable for usage in high-temperature environments. It remains structurally stable, retaining its strength and integrity even when subjected to intense heat. Additionally, aluminum exhibits a low coefficient of thermal expansion, resulting in minimal expansion and contraction in response to temperature fluctuations. This particular attribute enables aluminum sheet to maintain its shape and dimensions even when exposed to drastic temperature changes. Furthermore, aluminum showcases exceptional corrosion resistance, further augmented by the formation of a natural oxide layer on its surface. This oxide layer serves as a protective barrier against chemical reactions, safeguarding the aluminum from deterioration even in harsh environments or extreme temperatures. In conclusion, aluminum sheet demonstrates outstanding performance in extreme temperatures due to its high thermal conductivity, high melting point, low coefficient of thermal expansion, and excellent corrosion resistance. These properties establish it as a reliable choice for a multitude of applications, including the aerospace, automotive, and industrial sectors, where the ability to perform under extreme temperatures is of utmost importance.
- Q:Are the aluminum sheets suitable for manufacturing heat exchanger tubes?
- Yes, aluminum sheets are suitable for manufacturing heat exchanger tubes. Aluminum has excellent thermal conductivity, corrosion resistance, and is lightweight, making it an ideal material for heat transfer applications.
- Q:Are aluminum sheets suitable for aviation applications?
- Yes, aluminum sheets are highly suitable for aviation applications. Aluminum is a lightweight material with excellent strength-to-weight ratio, making it ideal for aircraft construction. It offers good corrosion resistance, high durability, and can withstand extreme temperatures. Additionally, aluminum sheets are easy to fabricate and can be formed into complex shapes, making them a preferred choice for various components and structures in the aviation industry.
- Q:Can aluminum sheets be perforated?
- Yes, aluminum sheets can be perforated. Perforation is a process that involves punching holes or creating a pattern of holes in a material. Aluminum is a versatile and malleable metal that can be easily perforated using various methods such as punching, drilling, or laser cutting. Perforating aluminum sheets can serve multiple purposes such as allowing for airflow, reducing weight, enhancing aesthetics, or creating filtration systems. The size, shape, and arrangement of the perforations can be customized to meet specific requirements and design preferences. Overall, aluminum sheets can be effectively perforated to enhance their functionality and visual appeal.
- Q:Can aluminum sheets be used for decorative ceiling panels?
- Aluminum sheets are indeed suitable for decorative ceiling panels. The versatility of aluminum as a material offers numerous advantages for this purpose. Its lightweight nature, durability, and resistance to corrosion make it an ideal choice for long-lasting use. Moreover, aluminum sheets can be easily molded and shaped into various designs, patterns, and textures, allowing for visually appealing ceiling panels. To enhance their aesthetic appeal and match the desired decor style, these panels can be finished with different coatings or finishes. Additionally, aluminum is a sustainable and eco-friendly material that can be recycled, making it an excellent option for those who prioritize environmental consciousness. In summary, the versatility, durability, and aesthetic potential of aluminum sheets make them an outstanding choice for decorative ceiling panels.
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Aluminum Panel Ceiling Sheet From China Wholesaler
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 100000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Related keywords





























