market price for carbon black /Rubber Antioxidant
- Loading Port:
- Qingdao
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 20000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Specifications
Rubber Antioxidant IPPD(4010NA)
1.101-72-4
2.Professional production factory
3.High quality
4.Used in the manufacture of tire
market price for carbon black /Rubber Antioxidant IPPD(4010NA)/ CAS No:101-72-4
(1)Product Name: Rubber Antioxidant IPPD (4010NA)
(2)Chemical Name: N-isopropyl-N'-phenyl-p-phenylene diamine
(3)Structure:
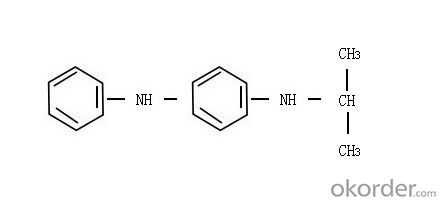
(4)Molecular Formula: C15H18N2
(5)Molecular Weight : 226.4
(6)CAS: 101-72-4
(7)Specification:
Index Name | Qualified Grade |
Appearance(eye measurement) | dark brown to dark violet pastilles |
Initial M.P.,°C ≥ | 70.0 |
Heating loss % ≤ | 0.30 |
Ash content % ≤ | 0.20 |
(GC)/Assay % ≥ | 95.0 |
Properties
Grayish purple to purple brown pastilles. Relative density is 1.14g/cm3. Soluble in benzene, acetone, ethyl acetate,
Carbon tetrachloride, chloroform, etc. Difficultly soluble in gasoline. Insoluble in water.
Application
It is an excellent and universal antioxidant used for natural rubber, synthetic rubber and latex. Better protective
performance of ozone, flex cracking, also the excellent protective agent for the heat, oxygen, light and the general
aging. It is able to inhibit harmful metals. It is mainly used in tires, rubber hose, belt and industrial rubber products.
Storage
The product should be sealed and stored in the dry, cool, well-ventilated area. Avoid exposure to direct
sunlight.
Packaging
25kg plastic woven bag lined with plastic film bag,kraft paper bag.
Following is our main products
Intermediate | Benzothiazole; MBT-NA | |
Rubber Accelerator | Thiazoles | MBT(M); MBTS(DM); ZMBT(MZ) |
Sulfonamides | CBS(CZ); MBS(NOBS); NS(TBBS); DCBS(DZ) | |
Dithiocarbamates | ZDEC(EZ); ZDMC(PZ); ZDBC(BZ) | |
Thiurams | TMTD(TT); TMTM(TS) | |
Guanidines | DPG(D) | |
Rubber Antioxidant | A(PAN); D(PBN); RD(TMQ); 4010(IPPD); 4020(6PPD); BLE | |
Antisorching Agent | PVI(CTP) | |
Rubber Vulcanizing Agent | DTDM; Insoluble Sulfur | |
Our Rubber Accelerator IPPD (4010NA) is produced by new technology---"enclosed clean
production techniques", located the forefront of industry in skill and output. Welcome to inquiry!
- Q: On the issue of chemical balance and catalyst
- The catalyst also changes the forward and reverse reaction rate.
- Q: Co and No form a chemical equation for Co2 and No2 under the action of a catalyst
- 2CO + 2NO == N2 + 2CO2
- Q: What about the chemical reaction of the catalyst if there is no catalyst?
- The catalyst used to heat and hold, the material will not react between.
- Q: What is a Catalyst?
- Catalysts are something that will speed up a reaction. Catalysts are usually acids but platinum catalysts are used in the industrial production of nitric acid (4 NH3 + 5 O2 ---4 NO + 6 H2O). Vanadium pentoxide is used as the catalyst in the industrial manufacture of sulfuric acid (S + O2 ---SO2. 2 SO2 + O2 ---2 SO3) The catalyst is used to make the reaction between sulfur dioxide and oxygen A LOT faster. Catalysts are also used in explosives. The most common is sulfuric acid (eg. nitroglycerin, TNT, nitrocellulose). Weaker acids like citric acid is used in the synthesis of HMTD, an organic peroxide. Hydrochloric acid is also a commonly used catalyst. Manganese dioxide can be used as a catalyst to generate oxygen when added to potassium chlorate or hydrogen peroxide. (2 H2O2 + MnO2 ---2 H2O + O2 + MnO2 (it is not necessary to include the catalyst in an equation, however). (2 KClO3 + MnO2 ---3 O2 + 2 KCl + MnO2).
- Q: How the catalyst accelerates the chemical reaction
- The effect of the catalyst on the rate of reaction and the effect of temperature on the reaction rate is fundamentally different. The catalyst can change the route of the reaction, reduce the activation energy of the reaction, increase the percentage of activated molecules in the reactants and increase the reaction rate.
- Q: Please make it simple because I need it for school and please give to examples for the second part Thanx :D
- A catalyst is a substance that speeds up the rate of a chemical reaction with itself being chemically unchanged at the end of the reaction. They are useful as they help to lower the minimum amount of energy needed ( also known as activation energy) to start the reaction. Hence, by lowering the activation energy of the reaction, they help to speed up the rate of reaction. For example, in the Haber process for the manufacture of ammonia, the catalyst iron is added to speed up the rate of reaction between hydrogen gas and nitrogen gas. Otherwise, the reaction would have proceeded much more slowly. Another example is the catalyst nickel used in the manufacture of margarine and vanadium (V) oxide for manufacturing sulfuric acid. As catalyst remain chemically unchanged after a reaction, they can be reused again and hence, they are required in minute amounts. An example is the washing powder used in washing clothes, they help to remove food stains by digesting the proteins in food. They can be reused after each reaction and hence, you do not need to add in the whole packet of washing powder but only a few spoonful.
- Q: Is there a catalyst for a chemical reaction?
- There may be many, but some of the catalytic effect of the catalyst is good, and perhaps some of the catalyst has not been found
- Q: Before and after the chemical reaction, the nature of the catalyst unchanged this statement right? Why?
- Chemical properties do not change better. Some properties of the catalyst may change before and after the reaction. If the experiment proves, the state of the catalyst before and after the reaction changes, and some changes from powder to powder.
- Q: Write a chemical formula in a chemical laboratory without the use of a catalyst for oxygen
- 2KMnO4 = Δ = K2MnO4 + MnO2 + O2 ↑
Send your message to us
market price for carbon black /Rubber Antioxidant
- Loading Port:
- Qingdao
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 20000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords

























