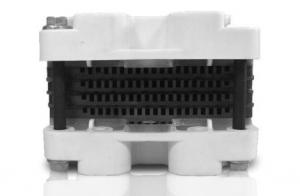
Specifications
*fuel cells are upgraded version fuel cell of PhyX series with better perfromance in stability and durability.
*fuel cell system is a highly integrated fuel cell system with compact design, who includes: fuel cell stack, fans, IC board and electromagnetive valve. 3000W is a suitable power for the mobile applications, for instance PC,
*motorcycle and backup power system.
Products characters:
1. Light weight and compact design
2. Low noise
3. Simple system and high reliability
4. Quick startup, good dynamic performance
5. Excellent environment adaptability;
6.Simple control and communication policy.
Packaging & Delivery
Delivery Detail:within 10 days