Amino Trimethylene Phosphonic Acid Best Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 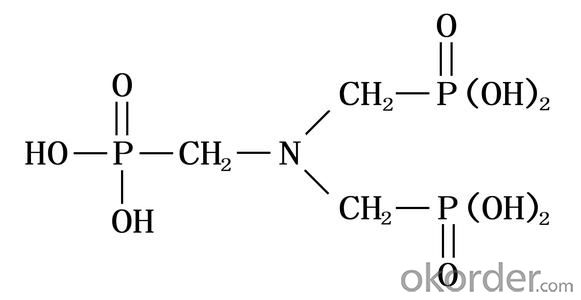
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q: What is the superiority of the catalyst compared to the stoichiometric reagent?
- Specificity: an enzyme can only catalyze one or a class of substrates, such as proteases that catalyze the hydrolysis of proteins into polypeptides;
- Q: Is it faster or slower? The What if you do not?
- The catalyst is divided into: positive catalyst and negative catalyst, positive catalyst accelerates the reaction rate, and negative catalyst slows down the reaction rate. Regardless of the positive and negative of the catalyst, it only changes the rate of the reaction, does not change the nature of the reaction, and the reaction, the chemical nature of the catalyst will not change!
- Q: Also, how is the catalyst affected by heat? Please answer all of the questions not just one of the three. THANK YOU!
- It denatures the catalyst because the rise in pH or amount of H3O+ ions. Temperature will also denature the catalyst if it's out of its optimum range.
- Q: How are the 4 characteristics of a catalyst (1. organic or inorganic 2. reusable 3. Highly specific, and 4. lowers activation energy) important in preforming life functions? please be as specific as possible, i understand that these are characteristic, i just don't understand why they're beneficial, other than the reusable and lowers activation energy one.
- Organic or Inorganic - the catalyst (enzyme) must be organic to be found in the cell. Catalysts speed up chemical reactions inside a cell and must therefore be organic to be a functioning part of the cell. Reusable - There are so many reactions that catalysts are involved in that it would be a waste for the cell if a catalyst could only last one reaction, especially if there are inhibitors and competition for the active site. Catalysts must be reusable in order to keep the cell functioning. Catalysts always remain unchanged after a reaction. HIihly Specific - Catalysts are only made to catalyze one specific chemical reaction. Their active site has proteins bonded in such a way that only certain elements can enter the active site and H bond with those proteins. The fact that they are highly specific maximizes the productiveness of the cell. And it ensures that the cell only has catalysts to reactions that it needs to be completed. It also ensures that the elements are correctly bonded with eachother. If any two elements could enter the active site, there is no guarantee that the correct product will be produced. Catalysts and Enzymes must be super highly specific in order to properly function. Lowers Activation Energy - The more energy a cell has to spend to catalye a reaction, the worse it is for the cell and the less ATP is has for other reactions. Catalyts hold the substrates together so there is less energy that is needed to have the two substrates react with eachother. Activation Energy is the energy that is needed to start a reaction. So the less energy used by the cell for reactions, the better for the cell. Hope this helps
- Q: How do I write about the ion equation?
- 4NH3 + 5O2 == 4NO + 6H2O
- Q: Where are they good catalysts and why?? THanks!
- A catalyst is a substance that speeds up a chemical reaction by providing an alternate reaction pathway with a lower activation energy. Catalysts appear not to take part in the reaction. Frequently, catalysts are not very reactive. Acids and bases, on the other hand, are very reactive. Acids (as H+) and bases ( as OH-) sometimes function as catalysts in some organic reactions. They appear to be catalysts because in the course of the mechanism H+ or OH- is regenerated.
- Q: The future direction of employment how, in what kind of units to do what work, how the closure rate? The
- Generally in the chemical plant to do engineering design engineers, the past few years, science and engineering graduates generally do not worry about work.
- Q: how does the amount of a catalyst affect reaction rate?
- A catalyst is actually a necessary part of the reaction. The catalyst is different on in that the catalyst returns to its original state when the catalyzed reaction completes. But that means that for each atom or molecule that goes through this reaction, there must be an atom or molecule of the catalyst to combine with. You could think of the catalyst as the buses that carry the reactants to their goal. The more buses, the faster the reactants reach their goal, but at the end, all the buses are empty, just like they started.
- Q: Is there a catalyst in the chemical shop?
- If it is manganese dioxide what is sold because it is not dangerous not expensive
- Q: What kind of chemical reaction requires a catalyst?
- For example, the system of ammonia, S02 oxidation into SO3
Send your message to us
Amino Trimethylene Phosphonic Acid Best Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches




















