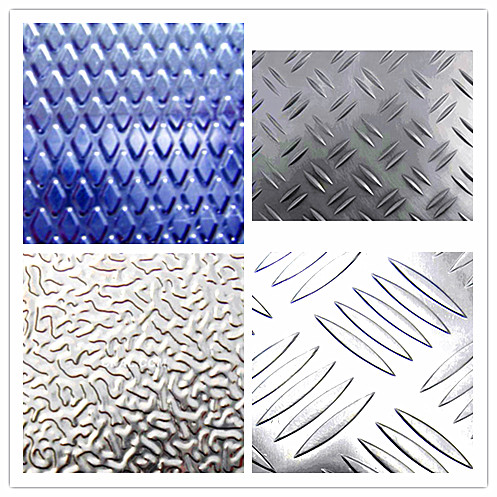
Embossed Aluminum Sheet Plate Manufactured in China
- Loading Port:
- Shanghai
- Payment Terms:
- TT or LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 9000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Aluminumtrade plate have great suctility, heat conductivity, anti-corrosion andmoisture resistance properties.
Alloy: AA1050, 1060, 1100, AA3003, 3005, 3015, 5052, 5754,5083,8011, etc
Temper: H14/16/18/22/24/32, HO etc.
Thickness: 0.2mm—100mm
Width: 100mm—2300mm (Can be slitted)
Application:
Anti-slipery purpose in vehicles,packing and appliance, decorative purpose
Business Items
1. Payment: T/T or LC at sight
2. Delivery time: 20 Days
3. Packing:
(1) EPE:protect aluminum.
(2) Plastic sheet:water-proof
(3 )Carton:anti-shock.4 Steel bar:fix
4. Quality: Mill Finish, Totally free from defects like White Rust, Roll Marks, Edge damage , Camber,Dents,Holes,Break Lines, Scratch

Dimensions can be produced according to your specifications,if you need any further details,please be free to tell us,we assure you of our best servieces.
If you are interested in our products, please contact us.

- Q:What type of aluminum plate does the traffic sign use? 1100?
- Formulated according to unused specifications and design requirements. May refer to the road traffic sign and the marking establishment standard execution.
- Q:Are 101 aluminum sheets magnetic?
- No, 101 aluminum sheets are not magnetic. Aluminum is a non-magnetic metal, which means it does not attract or hold a magnetic charge.
- Q:or aluminum ? and what other gases are released?
- Aluminium does not normally react with water because of a surface coating of the highly unreactive Al2O3 that forms on exposure to atmospheric oxygen. In the presence of strong base, however, this compound dissolves due to complexation by hydroxide, similarly to how silver chloride dissolves in ammonia. Al2O3(s) + 2OH-(aq) + 3H2O(l) ----- 2[Al(OH)4]-(aq) Once this has occured, aluminium metal, a very strong reducing agent, is exposed to water. 2Al(s) + 6H+(aq) ----- 2Al3+(aq) + 3H2(g) Even though the solution is alkaline, there is still a low concentration of H+ formed by the autoprotolytic dissociation of water. The Al3+ formed then reacts with hydroxide to produce more [Al(OH)4]-. Yes, the hydrogen gas is from the water.
- Q:I'm working on a costume that's entirely made of cardboard (it's a giant robot), and I was wondering What's the best way to1 turn Soda cans into aluminum sheeting2: attach the aluminum everywhere on the robot3. Attach the aluminum to the aluminumThank you in advance.
- Why not heavy duty aluminum foil glued to cardboard? that would look a lot better than patched up pieces of aluminum from tin cans. How would you connect the chunks of aluminum together? and remove the label. Note that soda cans have a coating of plastic on the inside. I don't see any easy way to attach the bits of aluminum from tin cans together, and remove the painted on labels. It would look just like that, pieces of cans glued together. In stead of cardboard you could use foam board which is lighter and more rigid.
- Q:Can aluminum sheets be used in automotive applications?
- Yes, aluminum sheets can be used in automotive applications. Aluminum is lightweight, corrosion-resistant, and has good strength-to-weight ratio, making it an ideal material for manufacturing automotive components such as body panels, engine parts, and structural components.
- Q:What is the typical elongation of aluminum sheets?
- The typical elongation of aluminum sheets ranges from 5% to 30%, depending on the specific alloy and temper.
- Q:Can aluminum sheet be used for automotive body-in-white applications?
- Certainly, automotive body-in-white applications can utilize aluminum sheet. Aluminum possesses the advantageous qualities of being lightweight, robust, and long-lasting, rendering it an optimal substance for automotive purposes. It presents a remarkable strength-to-weight ratio, which contributes to enhancing fuel efficiency and overall vehicle performance. Furthermore, aluminum excels in resisting corrosion, enabling it to endure harsh environmental conditions. Its malleability permits the creation of intricate and sophisticated designs, empowering manufacturers to fabricate automotive structures that are simultaneously light and sturdy. In recent times, the utilization of aluminum in automotive body-in-white applications has gained considerable popularity, primarily due to its ability to decrease vehicle weight and emissions while simultaneously maximizing safety and performance.
- Q:The real bumper/ reinforcement bar that is behind the plastic bumpers. Magnet don't stick to it and it don't rust which both types of metal are pretty much like that.
- Aluminum, stainless is too expensive and not ductile enough to be made into bumpers, especially of that complexity. As far as the magnetism, the magnet would only stick to low nickel stainless and its not likely that they would use 308 on a consumer vehicle.
- Q:how to connect copper pipe and aluminum sheet?
- it depends on the size and requirement, we can use ultrasonic wave rolling welding to connect copper pipe and aluminum sheet.for example, solar energy radiator-fan contains connection of copper pipe and copper sheet, ultrasonic wave rolling welding equipment is used here.ultrasonic wave welding of metals is suitable for the welding of copper to copper, copper to aluminum and aluminum to aluminum.
- Q:To an idiot, this may seem like a stupid question: its just aluminum. But it may not be.However seeing that I am only a high school student and don't have access to aluminum power (which is what I desire for an independent experiment) so I'd like to grind consumer grade aluminum foil down bases of Mohs scale of Hardness.My concern is that aluminum foil is Aluminum oxide which is a 9 (10 being diamond, 1 being talcum powder) and this would be horribly difficult to grind.If it is Alumina, that's only a 3.5.If it does happen to be aluminum oxide, can you think of a way to remove the Oxygen. Would nitric acid work.
- Foil will not be an element, it is a descriptor meaning a thin paper-like sheet of steel. Aluminum foil is constructed from pure sophisticated Aluminum which is an aspect. Given that Aluminum metallic is particularly reactive, the surface of the foil is without a doubt a skinny coating of Aluminum Oxide formed in reaction with air. This coating acts as a security towards additional corrosion. This corrosion resistance is why aluminum is the preferred steel foil constituent.
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Embossed Aluminum Sheet Plate Manufactured in China
- Loading Port:
- Shanghai
- Payment Terms:
- TT or LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 9000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Hot Searches
Related keywords































