Calcium Carbide with Competitive Price and High Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 22.5
- Supply Capability:
- 2400 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
1. Structure of Calcium Carbide Description:
CAS NO.:75-20-7
MF:CaC2
Grade Standard: Industrial Grade
Standard:GB10665-2004
HS Code:28491000
Purity: Gas Yield:295L/KG MIN
Packing&Delievery:50kg~100kg/IRON DRUM
All year Delievery
2. Main Features of Calcium Carbide:
This product is lumpy substance, its surface is a little deep gray, has slight nasty smell. It can produce acetylene gas when met water, it can burn when meets fire. The acetylene gas mix with air will form explosive gas (explosive range of acetylene gas in the air is 2.3% - 81%).
3.Calcium Carbide Images



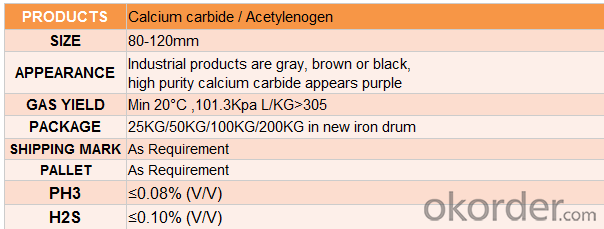
4.Calcium Carbide Specification
5.FAQ
Are you a factory or trading company?
----We are the import-export branch of the factory--CNBMGroup. There are production unit and sales unit in our Group. Production unit is only responsible for producing cargos. We are in charge of sales. Even there is a "trade" in our name,but actrually we are factories.
• What is the minimum order?
----One Metric Ton
• How can I get sample for testing?
----Kindly send us your address, we will send the sample to you.
- Q: Oxygen is not simple
- Yes. Because there is only one element of pure matter
- Q: What is the common substance oxide in junior middle school chemistry
- Elemental: chlorine, hydrogen, oxygen, nitrogen, sulfur, carbon, phosphorus, aluminum, potassium, sodium, magnesium, iron, silver, copper, zinc and so on.
- Q: Diamond, graphite is a simple element?
- Is the elemental, but the molecular structure is not the same. There is a chemical bond in the same element.
- Q: What is a compound?
- The compound is a pure substance consisting of two or more different elements. The different atoms that make up the compound must be present in a certain proportion, in other words, the compound has a certain composition regardless of the source. In daily life, sodium chloride, and distilled water (water), are common compounds. From these compounds, it is found that their properties are different from each other, the salt is composed of sodium atoms and chlorine atoms; sugar is composed of carbon, hydrogen and oxygen atoms; hydrogen in the combustion of oxygen reaction generated water. These facts indicate that two or more substances can react to produce a new substance, which is the compound. The nature of the new material is completely different from the nature of the substance. Usually chemically in this way to determine whether the nature of the compound is a compound. And if a pure can be broken down into two or more than the quality of the original quality of the compound. Such as molten salt, through the current, can be completely broken down into sodium and chlorine atoms, so the salt as a compound.
- Q: Diamond graphite c60 are simple elements do?
- Diamond, graphite, C60 are composed of carbon elements of the elemental, diamond, graphite is composed of carbon atoms, C60 is composed of molecules that C60 molecules, diamond and graphite due to the different arrangement of carbon atoms so there is a great physical properties Differences, the diamond is the natural existence of the most hardest material, graphite can be conductive to do the electrode, activated carbon and charcoal can adsorb toxic gases and pigments, carbon simple chemical properties similar to both flammability and reductive, combustion to produce carbon dioxide.
- Q: What are the black solid elements in junior high school chemistry?
- C (charcoal, graphite)
- Q: Does the mixture have a simple substance? What is it?
- Such as red phosphorus and white phosphorus, the molecular formula is P, but the structure is not the same, even if the mixture is oxygen and ozone, the molecular formula is not the same, but are composed of O elements
- Q: Is chlorine gas simple?
- Chlorine is simple.
- Q: What is the element?
- A substance consisting of atoms of an element that is more stable in free form. Such as oxygen (O 2), chlorine (Cl 2), sulfur (S), iron (Fe), and the like. Elementary and element are two different concepts. The elements are collectively referred to as atoms having the same number of nuclear charges (protons). An element may have several elements, such as oxygen (O 2), ozone (O3) tetramerox (O4, a new type of oxygen molecules synthesized by a scientist in Italy). Although any of these substances are simple, because they are pure elements of the same element, but oxygen and ozone together is not pure, they are the same kind of elements of the mixture.
- Q: What is HD?
- HD should be a special element, solid H2 is the molecular crystal. At room temperature, HD for the gas, does not belong to the crystal.
Send your message to us
Calcium Carbide with Competitive Price and High Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 22.5
- Supply Capability:
- 2400 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches

























