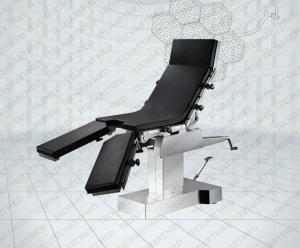
super deluxe multi-functional medical surgical table with C arm
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Super deluxe multi-functional medical surgical table with C arm
ET700 electromotion facilitates the various adjustment of positions,intellectualized reposition enables the table a quick
return to horizontal position.Automatically lockable remote control in case of wrong operation.
Large-scale elevation makes it easier for C-arm fluoroscopy examination .
With built-in kidney bridge.suits for kidney operation .
One button reposition function,designed and made specially for X-raying and C-arm use.
This is also an excellent orthopedics table,which can use with Traction
Features:
1) electro- hydraulic adjustment
2) ergonomic comfort
3) cost- effectively
4) muti-performance
5)advanced longitudinal shift
- Q: Medical equipment company operating range
- Business scope is the business scope of business activities, should be approved within the scope of business activities. According to the provisions of the State Administration for Industry and Commerce, "the scope of business registration management," the provisions of the company's scope of business should refer to the national economic industry classification standards and the relevant provisions. 4, apply for general business projects, the applicant should refer to the "national economic industry classification" and the relevant provisions of the choice of one or more types of business, according to the law directly to the enterprise registration authority for registration. The business scope of the business scope of the business should be consistent with the industry name of the enterprise name. The business scope of the enterprise shall be registered by the enterprise registration authority according to the application of the investor or the enterprise. The scope of business shall be consistent with the provisions of the articles of association or the partnership agreement. The applicant shall apply to the examination and approval authority in accordance with the laws, administrative regulations and the State Council, and upon application, it shall apply to the enterprise registration authority for approval through the approval documents and documents. The enterprise registration authority shall, in accordance with the approval documents, documents and registration of the examination and approval authority, operate the project.
- Q: How long can the wound be wiped after surgery?
- According to the size of surgery knife ~ ~ ~ generally in the half months to January after
- Q: Beijing medical equipment company which?
- Too much. For example: Beijing Huajie Hao Technology Development Company, Beijing Li Bell Medical Devices Co., Ltd., Beijing Li Dakang Technology Development Company, and so on
- Q: TST is a technique or a surgical instrument
- TST is (Tissue-selectingTherapystapler) minimally invasive surgery, also known as selective hemorrhoid mucosal anastomosis, was anorectal clinic as the most secure minimally invasive technology, is the recent development of PPH on the basis of a new type technology.
- Q: After the back of the tumor surgery doctors explain a lot, forget some! Twelve days after the stitches, three days later do not remember what. There is a bandage like a bandage on the back. Three days after the bandage should be removed it. And the meaning of the stitches is that the skin has been exposed to the outside, the doctor in the pull line? Ask the specific meaning of the stitches, to the regular hospital but also spend money? Now have to hang a number to 42!
- Three days should dressing, that is, re-disinfection dressing, 12 days the whole can be stitches, casually find a small hospital clinics can be stitches
- Q: Surgical equipment after the initial soaking disinfection with what the best disinfectant
- Glutaraldehyde is a highly effective disinfectant. Because of the bactericidal spectrum is wide, the bactericidal spores are reliable, the bactericidal effect is less affected by the organic matter, the metal is corrosive and so on. It is suitable for the disinfection and sterilization of the heat-resistant medical equipment and precision instruments, especially the endoscope Disinfection and sterilization. However, since glutaraldehyde is potentially toxic to the human body and is irritating to the skin and mucous membranes, some people are actively looking for alternatives to sterilization for endoscopes and other medical devices. ???????The new dilution can be used to wash hands before surgery (0.05 to 0.1%, which can be used to remove the clothes, and it is easy to be preserved. It is a kind of anti- Soak for 5 minutes), skin disinfection and fungal infection (0.1%), mucosal disinfection (0.01 ~ 0.05%), disinfection equipment (0.1% solution in boiling for 15 minutes and then soaked for 30 minutes) Or other synthetic detergent used to avoid the use of aluminum containers, disinfection of metal equipment need to add 0.5% sodium nitrite rust, should not be used for cystoscopy, ophthalmic equipment and synthetic rubber disinfection. Gram-negative bacilli and intestinal The virus is weak and has no effect on Mycobacterium tuberculosis and spores.
- Q: Are medical tweezers a medical device?
- Medical tweezers are medical device management Due to the many types of medical tweezers, tweezers of different uses may belong to different management categories, such as small vascular tweezers in the "basic surgical instruments", no injury tweezers, tissue tweezers, plastic tweezers, needle tweezers, ), Pull hair tweezers, towel tweezers, dressing tweezers, anatomical tweezers and so on are class Ⅰ medical equipment management;
- Q: What is the principle of postoperative wound pressure
- After surgery, the wound must have different degrees of bleeding, bleeding, and even the formation of hematoma or the formation of scrotal hematoma. The presence of these factors will affect the wound healing, is not conducive to the complete cure of abdominal hernia, and the presence of bleeding and hematoma, prone to wound infection, induced hernia recurrence, leading to surgical failure. Through the use of sandbags or other heavy objects to oppress the wound, can effectively reduce the wound bleeding, prevention of wound exudation and lead to hematoma formation and the occurrence of scrotal hematoma, for the success of surgery to create favorable conditions.
- Q: What are the equipment and names of the operating room?
- Equipment cabinet, medicine cabinet, anesthesia cabinet, writing desk, anesthesia clock, hanging tower, shadowless lamp
- Q: Say 10 kinds of medical device name
- Serial number Name An example of a product name category 1 Medical suture needle (without line) Medical suture needle (without line) II 2 Basic surgical knife Surgical knife handle and blade, leather knife knife, wrist stripping knife, lancet, blade, shaving knife, dander scraper, pick knife, front knife, pedicure Knife, repair a knife, scalpel I
Send your message to us
super deluxe multi-functional medical surgical table with C arm
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
OKorder Financial Service
Similar products
Hot products
Hot Searches
Related keywords