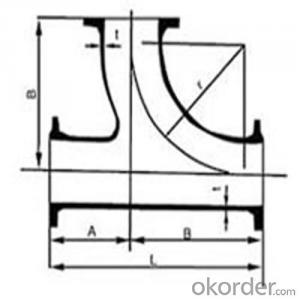
ductile cast iron fittings--bends
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
Quality Product, Order Online Tracking, Timely Delivery
OKorder Financial Service
Credit Rating, Credit Services, Credit Purchasing
You Might Also Like
Packaging & Delivery
| Packaging Detail: | export packing |
| Delivery Detail: | 30 Days |
Specifications
1.near to 20 years manufacturing and exporting experiences.
2.ISO2531,BSEN545 standard.
3.DN80-1800,Centrifugal casting,K12/14
Ductile iron cast fitting in iron fitting zinc&bitumen
1.Internally:cement lining conform to ISO4179(portland cement/sulphate resistant cement/high-alumina cement).
2.Externally:bitumen or epoxy (70 um or more) coating base on zinc (130g/m2 or more) conform to ISO8179
3.Certificate:ISO9001,ISO14001,SGS,NSF,WRAS
- Q:I hear people say that but how? A flat iron straightens your hair.
- A copper line is 1000 times better than black iron;black iron rusts at the plumbing supply house , much less under-ground; call your local codes department and they will answer any questions!
- Q:im doing a school assessment on iron and i need your help pleaz :)
- Lack of iron can lead to anemia
- Q:Im looking at buying some new irons, i have a 16 handicap and i wanted some feedback on clevelands gold and red irons and also the hibore ironsat 16 handicap will it be a mistake to go with the red irons?
- I have tried t he CG Gold irons. They are solid irons. I like Cleveland clubs. I do think the Mizuno MX-23s are easier to hit out of the rough. The CG Red is for better players. Many cleveland tour pros it. On the other hand, I think you are too good for the Hibore irons. It depends why you are a 16 handicap. If you are 16 because your ball striking is good but putting and short game, are poor you can consider the red irons. But if are a 16 because of poor ball striking, go with the gold. You can get the Gold Combo Set (3,4 iron is Hibore, 5-PW are CG Gold)
- Q:i have to compare the uses of stainless steel to iron
- Iron is generally used for decorative backyard furniture, magnets and simple metal structures. It rusts easily, so iron must be painted to protect it.
- Q:I’m taking supplements how long does it takes to get an over dose of iron? I’m taking at between 200% and 300% a day. Because, I read that some of the iron taken doesn’t actually get absorbed by the body and also believe that I might be deficient. So what I’m asking is how long would one continue taking iron before it becomes detrimental?Thanks
- Iron OD symptoms are irritation and ulceration of the stomach lining. Symptoms are nausea, vomiting and abdominal pain 20 minutes after ingesting. Then it damages the stomach ,liver, kidneys, lungs, blood vessels and brain. Iron overload can damage the brain and heart and lead to heart attack or stroke. Most people have an over abundance of iron rather than too little iron. B vitamins can help reduce iron levels. I would suggest you get tested for low iron instead of treating yourself, you could cause your own death if you don't know what you are doing. Please stop and check with a Dr before you take more iron, it is very dangerous to your health to overdose on iron.
- Q:Iron HCL react together according to Fe + 2HCL FeCl2 + H2Depending on the reaction conditions the product may be (i) solid, anyhydrous iron II Chloride (ii) an aqueous solution of iron II Chloride, or (iii) crystals of iron II chloride-4-water. get suitable state symbols and make any other modifications to the basic equation to represent the following.1) passing dry hydrogen chloride gas over heated iron to give anyhydrous iron (II) chloride2) Dissolving iron in hydrochloric acid to give a solution of iron (II) chloride3) dissolving iron in hydrochloric acid and then crystallising the solution to give iron (II) chloride-4-water
- This is a bit of an odd question. Basically you want to classify all your chemicals as either being solid, liquid, gaseous or aqueous. Aqueous means that it is in solution. 1) This one involves using gaseous hydrogen chloride which reacts with solid iron to form solid iron(II) chloride and hydrogen gas (anhydrous means without water). Fe(s) + 2HCL(g) FeCl2(s) + H2(g) 2) Here we have solid iron being dissolved in a solution of hydrochloric acid (which is basically the hydrogen chloride gas dissolved in water). Here we see that the iron starts solid and is reacted with the aqueous hydrogen chloride/ hydrochloric acid to form a solution of iron(II) chloride. Fe(s) + 2HCL(aq) FeCl2(aq) + H2(g) 3) This is basically the same as number two, except with the solution of iron(II) chloride, we evaporate the water which leaves some in the crystal structure of the solid iron(II) chloride-4-water. Note that you write water as being a liquid and not being aqueous. 4H2O(l) + FeCl2(aq) 4H2O.FeCl2(s) I hope this is what you need and it helps.
- Q:Who would win and why?I say Iron Fist!
- Iron Fist would win easily. With his chi power, Green Arrow's various arrow would not withstand against Iron Fist's chi power.
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
ductile cast iron fittings--bends
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
Quality Product, Order Online Tracking, Timely Delivery
OKorder Financial Service
Credit Rating, Credit Services, Credit Purchasing
Similar products
New products
Hot products
Related keywords