Calcium Carbide with Cheap Price and High Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 22.5
- Supply Capability:
- 2400 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
1. Structure of Calcium Carbide Description:
CAS NO.:75-20-7
MF:CaC2
Grade Standard: Industrial Grade
Standard:GB10665-2004
HS Code:28491000
Purity: Gas Yield:295L/KG MIN
Packing&Delievery:50kg~100kg/IRON DRUM
All year Delievery
2. Main Features of Calcium Carbide:
Calcium carbide is one of the basic raw material of organic synthesis industry.It is also an important raw material of acetylene industry.
(1)The reaction of calcium carbide with water, producing acetylene and calcium hydroxide.[CaC2 + 2 H2O → C2H2 + Ca(OH)2].
This reaction was the basis of the industrial manufacture of acetylene, and is the major industrial use of calcium carbide.
eg.synthetize rubber,synthetic resin,acetone,ketene and carbon blace.Meanwhile oxygen-acetylene flame is widely used in metal welding and cutting.
(2)Heating powdered calcium carbide and nitrogen to producing calcium cyanamide,which can react with sodium chloride producing black cyanide.
black cyanide is used in gold mining and non-ferrous metal industry.
3.Calcium Carbide Images



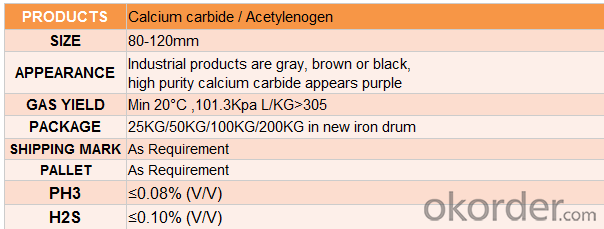
4.Calcium Carbide Specification
5.FAQ
Are you a factory or trading company?
----We are the import-export branch of the factory--CNBMGroup. There are production unit and sales unit in our Group. Production unit is only responsible for producing cargos. We are in charge of sales. Even there is a "trade" in our name,but actrually we are factories.
• What is the minimum order?
----One Metric Ton
• How can I get sample for testing?
----Kindly send us your address, we will send the sample to you.
- Q:Oxygen is not simple
- Yes. Because there is only one element of pure matter
- Q:Does the mixture have a simple substance? What is it?
- How can we say that the mixture is simple? Simple substance is pure material pure matter only one molecule and a stuff contains two or more molecules is the mixture problem is clearly unreasonable Well
- Q:What are the black solid elements in junior high school chemistry?
- Carbon (C), iron powder (Fe), ferrous oxide (FeO), charcoal (carbon black, activated carbon), copper oxide (CuO), manganese dioxide (MnO2), iron tetraoxide (Fe3O4)
- Q:Elementary is a few years of course
- Elementary is the first three years of chemistry
- Q:What is the difference between chemical monomer and elemental
- And the element is composed of the same element of pure matter. An element is called a free state of an element in the presence of an element. In general, the nature of the element is closely related to the nature of its element. For example, the metal properties of many metals are obvious, then their elemental reducibility is very strong. Different elements of the element, the nature of the differences reflected in the structure of the most prominent. In contrast to elemental matter, a substance consisting of multiple elements is called a compound
- Q:What is the chemical formula of iodine element?
- Iodine, which is located in the periodic period of the chemical element in the 5 cycle. Series VIIA is one of the halogen elements. 1811 French pharmacist Kutwa first found that iodine iodine. Elemental iodine was purple black crystal, easy sublimation, after sublimation easy to Ninghua. Toxic and corrosive. Iodine case of starch will become blue and purple. Mainly used for pharmaceuticals, dyes, iodine, test paper and iodine compounds. Iodine is one of the essential trace elements in the body. The total amount of iodine in healthy adults is 30 mg (20 to 50 mg), and the national standard for adding iodine to salt is 20-30 mg / kg.
- Q:What is the elemental element
- Should be a simple element and element it Elemental matter must be pure matter composed of the same element, the mixture can not be simple. An element is called a free state of an element in the presence of an element. The element is the general term for the same class of atoms with the same number of nuclear charges (protons)
- Q:Diamond, graphite is a simple element?
- Is the elemental, but the molecular structure is not the same. There is a chemical bond in the same element.
- Q:What are the two kinds of prime
- Metal or non-metallic. Either a single molecule or a multi-molecule. Depending on the different points.
- Q:Is chlorine gas simple?
- Molecular formula Cl2
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Calcium Carbide with Cheap Price and High Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 22.5
- Supply Capability:
- 2400 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products































