TCCA In Water Treatment Chemicals Special for Swimming Pool
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 22 m.t
- Supply Capability:
- 1800 m.t/month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
TCCA 90% Powder, Granular, Tablets
Introduction:
CNBM –TCCA White tablet with irritant chlorine odor. Slight solute in water. TCCA is the organic compound with the formula (C3Cl3N3O3). It is used as an industrial disinfectant, bleaching agent and a reagent in organic synthesis. This white crystalline powder, which has a strong "chlorine odour," is sometimes sold in tablet or granule form for domestic and industrial.
Specification:
Chemical Name | Trichloroisocyanuric Acid |
Molecular Formula | CONCL3 |
CAS Number | 87-90-1 |
Avaliable Chlorine %min | 90.00 |
Moisture content %,wt,Max | 0.30 |
PH Value (1% solution) | 2.7~3.3 |
Solubility 25℃ Water | 1.2g/100g |
Solubility 30℃ Acetone | 36g/100g |
Specific Gravity | 0.95(light)/1.20 |
Granular Particles Size
Mesh | 5~8 | 8~30 | 20~40 | 20~60 |
Tablets Forms
Weight | 200 gram | 150gram | 100gram | 50gram | 30gram | 20gram | 15gram | 10gram |
Diameter(mm) | 76 | 70 | 50 | 42 | 30 | 30 | 30 | 30 |
Height(mm) | 25 | 21 | 26 | 27 | 22 | 16 | 12 | 8 |
Multi-Functions
We made multifunctional tablets according to customers’ needs. For Instance, we press TCCA 90% granular with chemicals such as Boric Acid,. Sulfate Copper, Sulfate Aluminum And PAC.
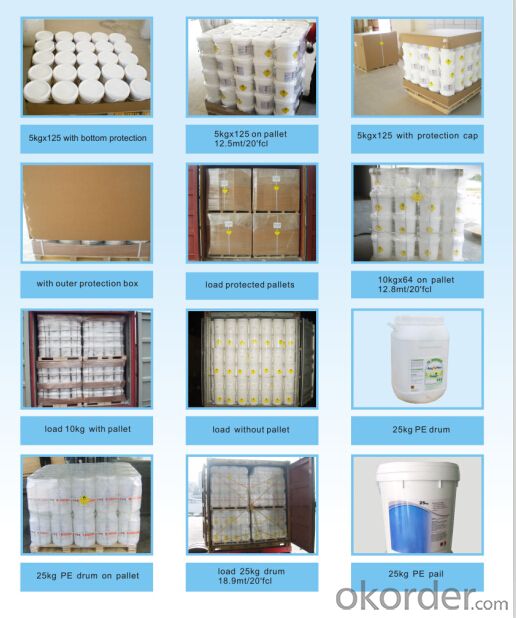
Packing:
Granular& Powder:
50KG PLASTIC DRUMS/ FIBER DRUMS.
25KG PLASTIC DRUMS/FIBER DRUMS.
1000KG BIG BAGS.
Or any other packages suggest by customers.


- Q:What is the microcosmic principle of the catalytic reaction in the chemical reaction?
- The catalyst reduces the activation rate of the reactants by increasing the reactant density of the reaction conditions and making the chemical reaction easier.
- Q:What is the difference between a catalyst and an inducer in a chemical reaction?
- The catalyst does not participate in the reaction, but only the carrier of the reaction; the inducer will participate in the reaction
- Q:What is the catalyst condition in the chemical equation?
- I studied three methods ~ word which can be used to give me a mailbox to the word text to pass you ~
- Q:I have just spent CAN$550 to replace a catalyst converter at one end of the muffler. The repairman said I need to replace the oxygen sensor very soon, otherwise, the C.C. will be gone again. Is that true... can someone confirm this for me. Thanks.
- ok its not catalyst converter its catalytic converter. next i have not read one answer that is correct a couple are close but no cigar. ok the 02 sensor senses the oxygen content in the exhaust gasses. using the oxygen content it can determine if the engine is running to rich or to lean. the ecm (computer) wants to keep the engine at optimum fuel mileage or keep it stoichometric meaning fuel to air ratio of 14.7:1 the ecm can adjust fuel air ratios by changing the pulse width and the duty cycle or on off time of the injector.it also uses several other sensors to help it like intake air temp (iat) coolant temp (cts) mass air readings if equipped (MAF) or manifold absolute pressure (map) so its not just the oxygen sensor it can make adjustments as need. anyways the oxygen sensor uses a .1 to .9 volt range signal anything below .450 is lean anything above .450 is rich and .4 to .5 is perfect. but ur engine constantly changes and the 02 readings change constantly new cars can take readings as fast as one or two times a second older cars are alittle slower. anyways only replace the 02 sensor if the car is telling u its bad meaning u have a check engine light on and a code that says P 0 . . . bank one sensor one ckt malfunction or bank 2 sensor 1 ckt malfunction u get the idea unless that happens ur 02's are good leave them alone. when they do go bad ur car will run rich and puke alittle black smoke out the exhaust and ur car will smell like rotten eggs. NOW the cat its job is to heat up to 600 + degreees F and burn unburnt fuel from the combustion cycle of the engine when an engine does not burn all its fuel its running rich and bad fuel milage occurs. if the 02 sensor goes out it can heat the cat red hot as the extra fuel being burnt inside it can become extremely hot. most 02 sensor for most vehicles run around 60 bucks not expensive. a car can have 1 to 4 of these and YES if bad and left for too long and can ruin a cat converter.
- Q:Also, how is the catalyst affected by heat? Please answer all of the questions not just one of the three. THANK YOU!
- It denatures the catalyst because the rise in pH or amount of H3O+ ions. Temperature will also denature the catalyst if it's out of its optimum range.
- Q:Before and after the chemical reaction, the nature of the catalyst unchanged this statement right? Why?
- Only catalyze, do not participate in the reaction! The
- Q:Exemplify the use of green catalysts in green chemistry
- How can a green catalyst, they do not react
- Q:What are the examples of chemical catalysts used in life?
- The use of new synthetic materials makes life more comfortable. Only wood, sand and grapes are natural building materials, but they need to be combined and protected with synthetic chemicals. Cement is a chemical product, As the adhesive used in the laminate and the metal used in the nail are chemical products, the glass is made by the chemist, and the improved product, such as heat-resistant glass (trade name Pyrex glass), becomes more tough. Paint is chemist design and creation, and many modern solid materials are also the same. Plastic is synthetic, they are used in kitchen and bathroom utensils, also used in the name of the product called Formica bakelite and its related materials, beverage bottles, Cutlery and utensils. Porcelain is made by chemists and used in kitchen and bathroom sinks and other fixtures. Metal is made of chemical changes made from ores. Aluminum was once a laboratory treasure, but used An electrochemical method, which now can be easily made from alumina, at least a portion of the carpet and decorative fabric used for the use of synthetic fibers and synthetic dyes to color. Freezer and air conditioner with special chemicals as coolant ; Gas and gas stoves can be used syngas or natural gas, the combustion process is still the chemical change.Our room with gas or oil industry to produce fuel to heat, this fuel is from the natural crude oil refining and chemical We have made use of synthetic chemical products and materials made in the chemical processing industry, such as plaster or wall panels, outer panels and roof panels, as well as tiles and carpets, to heat our buildings. The stove itself and the distribution of heat The pipes are made of chemical products - metal, insulating materials and ceramics. The current enters the home through the copper wire of the outsourced insulator, both of which are products of the chemical processing industry
- Q:Characteristics and types of catalysts?
- Catalysts don't undergo any change. and types of catalysts - 1) Homogeneous Catalysts ( Having same phase that of reactant, product i.e. reactant and product and catalysts all are either liquid or gas or solid.). 2) Hetrogenrous Catalysts (Different Phase than that of reactant and product. 3) Autocatalysts (reaction proceed catalysed as product is formed or product catalyse the reaction.)
- Q:Will the catalyst be able to increase the rate of chemical reactions?
- In general, the catalyst has a positive catalyst (i.e., accelerates the reaction rate) and a negative catalyst (i.e., reduces the reaction rate), and generally does not specifically refer to both the positive catalyst.
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
TCCA In Water Treatment Chemicals Special for Swimming Pool
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 22 m.t
- Supply Capability:
- 1800 m.t/month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Related keywords



























