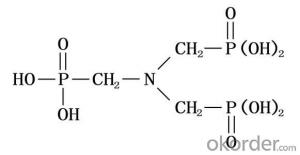
Methylcellulose Medium
Methylcellulose Medium Related Searches
Ks Standard Sliding Gate Track Global Terminal Gate CamHot Searches
Methylcellulose Medium Supplier & Manufacturer from China
Okorder.com is a professional Methylcellulose Medium supplier & manufacturer, offers integrated one-stop services including real-time quoting and online cargo tracking. We are funded by CNBM Group, a Fortune 500 enterprise and the largest Methylcellulose Medium firm in China.Hot Products
FAQ
- Is the catalyst considered a chemical reaction?
- But in fact the catalyst is in the process of the whole process of the catalyst involved in the reaction of the consumption of the catalyst but eventually produced a catalyst equivalent to no reference
- Will the catalyst decompose during the reaction between two substances? Exp:the decomposition of hydrogen peroxide.Will the manganese 4 oxide decompose?
- Catalysts are not used/destroyed in any reactions, it merely speeds up the process by lowering the reaction activation energy. It functions by being able to weaken or break the required bonds necessary in the chemical reaction (thus lowering activation energy) through temporary and weak bonding to form a complex. In this case the H2O2 molecule will bind with the MnO2 molecule due to the complimentary sites (thus forming a complex) to weaken the bonds for decomposition, but after decomposition the products (oxygen and water molecules) break off from the catalyst (as there are no more complementary sites with them) thus the catalyst will not be destroyed.
- What is the analytical principle of chemical adsorbents? How about the number of active catalyst centers tested?
- What do you mean by the chemical adsorber? BET is the use of the surface of the uneven force field, but the inert gas at low temperature in the surface adsorption. TPD, TPR is the number of active centers that can be measured by the technique of desorption and reduction between specific gases and catalysts as the temperature increases. If the active site is a reduced position, H2-TPR can be used. If the active site is acidic, NH3-TPD can be used, but also the method of alkali titration.
- It's a GCSE Chemistry questionI just need to know why there are so many
- Many important chemical reactions require inputs of energy to proceed. If a catalyst is present less energy will be required to complete the reaction. Catalysts are substances that are mixed in with materials that are to be reacted, but they themselves do not, in the end, change chemically. They establish a local environment that promotes one or more chemical reactions to take place. A catalyst is important in many industrial processes. Sulfuric acid, which is used to produce batteries, detergents, dyes, explosives, plastics, and many other produces, is commonly produced using a catalyst called vanadium oxide. Ammonia, a primary component of many fertilizers, could not be produced economically without the use of iron oxide which speed up the reaction. The process of catalyst also affects the state of our global environment. Automobiles use catalytic converters to treat exhaust. The metals platinum and palladium facilitate the chemical conversion of noxious gases to more inert forms, greatly decreasing the environmental impact of combustion engines. Probably the most important impact of catalyst is on life itself. All important biochemical reactions are catalyzed by molecules called enzymes. Most enzymes are proteins which catalyze specific reactions within cells. Some examples include polymerases, which synthesize DNS and RNA, peptidases, which digest protein, and ATP synthases, which produce energy for the many different cell activities.
- How do I write about the ion equation?
- 4NH3 + 5O2 == 4NO + 6H2O
- What are the methods of catalyst characterization?
- Physical means, is commonly used detection means, infrared, ultraviolet, electron microscopy, X-ray diffraction, nuclear magnetic, etc., of course, including a variety of conventional inability analysis.
- Chemical questions: "CO2 and H2 in the catalyst conditions have a reaction, the reaction of the chemical equation is"
- CO2 + H2 = CO + H2O (conditions: catalyst, generally requires heating, and reversible)
- how can you tell when a substance serves as a catalyst?
- The purpose of a catalyst is to provide an alternate pathway for a reaction to proceed, often one with a lower activation energy, such that the reaction will generally proceed faster. The key to catalysts if they they are NOT consumed by the reaction in the end (they may be consumed in an intermediary step, but if so, a subsequent step will recreate the catalyst). In other words, catalysts do not actually participate in the reaction, so they may be reused when the reaction has completed.