Sodium Dichloroisocyanurate SDIC For Water Treament
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 9000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Sodium Dichloroisocyanurate SDIC
Introduction:
SDIC White powder or grain with chlorine odor . It is a strong oxidant and chlorate agent and can dissolved in water easily . Its aqueous solution
assumes weak acidity and the active chlorine in its dry products lose little when it is stored for a long time at the atmospheric temperature .
Specification:
Chemical Name | Sodium Dichloroisocyanurate | |
Molecular Formula: | C3O3N3HCL2NA | |
Molecular Weight: | 220.96 | |
CAS Number: | 2893-78-9 | |
Product | 60% | 56% |
Available chlorine(%,min) | 60 | 56 |
Moisture content(% max) | 5 | 8 |
PH Value(1% solution) | 6-7 | 6-7 |
Particles Size:
Mesh | 5~8 | 8~30 | 20~40 | 20~60 |

Main usage:
this products can effectively kill various germs, fung uses and viruses, specially A&B type hepatitis viruses. It is effective on killing algae,
decolorizing cleaning water or bleaching .It can be widely used for epidemic prevention, livestock farming , industry and agriculture.

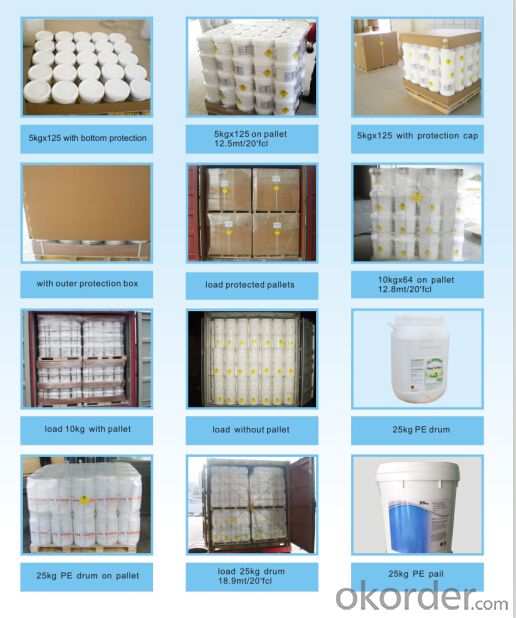
Package:
50KG PLASTIC DRUMS/ FIBER DRUMS.25KG PLASTIC DRUMS/FIBER DRUMS. 1000KG BIG BAGS. Or any other packages
suggest by customers.

- Q:Chemical reaction plus catalyst on the △ H no effect
- Since the addition of the catalyst only accelerates the reaction process and does not have an effect on the reaction product and the reactants
- Q:What is the principle of catalyst reaction rate in chemical reactions?
- Whether the chemical reaction can be carried out according to the change of free energy, but only according to the change of free energy can not determine whether the reaction can be completed, because the chemical reaction is also completed by the reaction of the energy barrier, that is, if the reaction energy barrier is high, To provide some energy, across the barrier, to complete the reaction. The energy barrier is called activation energy. And the role of the catalyst is to reduce the activation energy, so that in a relatively harsh environment, chemical reaction occurs.
- Q:High school knowledge __ teacher do not know right!
- Nothing to do, but with the percentage of activated molecules, is proportional to
- Q:If possible can anyone give me information on the active site, substrates, products, and the energy of activation as part of the answer?Responses greatly appreciated! Thankss! 10pts to best answer!
- Catalysts help shift the equilibrium of a reaction to one that is more favorable. They allow a naturally occurring reaction that may be extremely slow to progress faster or an unfavorable reaction to proceed forward. During the process catalysts are recycled, which means that at the catalyst is the same compound in the beginning and the end of the reaction, although during intermediate steps catalysts can change conformation. Catalysts shift the equilibrium of a reaction by lowering the activation energy of a reaction, which is the energy barrier which must be overcome in order for the reaction to proceed in a desired direction. This can be achieved in several ways such as providing favorable thermodynamic conditions for a reaction or creating intermediates which react more favorably to create the products. Inside the cell a lot of chemical reactions are either too slow to proceed naturally or are simply unfavorable. Catalysts help overcome those barriers. The substrate is the part of the reaction which gets transformed into the products after binding to the active site of the protein.
- Q:Does the nature and quality of the catalyst itself change before and after the chemical reaction?
- Will not change! In fact, the catalyst in the chemical reaction is to participate in the reaction, but in the reaction it not only participate in the reaction, but also generated, and the amount of reaction and the amount of equal, the total amount of the same, this process is more complex, But sometimes the problem will appear, but the information will be very clear, will not affect the problem!
- Q:I dont know what it is but when i open up my computer it comes up and it says that its not working? so i really dont know what to do.
- ATI Catalyst Control Centre is a control program for ATI graphics cards. If you have an ATI graphics card, go to the ATI website and download and reinstall the program.
- Q:describe a biological catalyst?
- A biological catalyst is a subclass protein called an enzyme. Enzymes are biological catalysts that increase the rate of chemical reactions. A catalyst does the following: 1) Increases the rate of reaction 2) Is not itself changed at the end of the reaction 3) Does not change the reaction or its final result The amount of energy required for this reaction is called activation energy. The enzyme lowers the activation energy necessary for the reaction to take place, thus speeding up the process.
- Q:The addition of the catalyst has no effect on the chemical equilibrium of the movement
- The essence of chemical equilibrium is a dynamic equilibrium. Under certain conditions, the equilibrium constant of the reaction is a certain value. The role of the catalyst is to reduce the activation energy required for the reaction, increase the number of activated molecules, and increase the number of molecules per unit time , But the positive reaction is positive for the reaction, so do not change the speed.
- Q:Just something I've always wondered about...
- transition okorder /... for ex-- X (one reactant) + catalyst(transition element) ------X.catalyst(intermediate unstable compound) X.catalyst + Y (other reactant) --------XY(product) + catalyst how the change in oxidation state of transition elements helps the reacton through the formation of intermediates may be seen from reaction in between SO2 and O2 to form SO3 in presence of V2O5 ... V2O5 + SO2 ------V2O4 + SO3 2V2O4 + O2 ------2V2O5 in the above reaction vanadium changes its oxidation state from +5 to +4 and again to +5.. another example is reaction in between iodide and persulphate ions in presence of Fe(III) as catalyst... 2I(-) + S2O8(2-) ---------I2 + 2SO4(2-) (Fe(III) is present as catalyst) the reaction is believed to take place as follows: 2Fe(3+) + 2I(-) ------2Fe(2+) + I2 2Fe(2+) + S2O8(2-) ------2Fe(3+) + 2SO4(2-) (3)in number of cases transition elements provide a suitable large surface area with free valencies on which reactants are absorbed ...as a result concentration of reactants on surface of catalysts increases..hence rate of reaction increases...this is known as adsorption theory.... according to adsorption theory : there are free valencies on surface of solid transition metals because of the incomplete d-subshelll.. so the mechanism of catalysis involve followin five steps: (1) diffusion of reactant molecules towards surface of catalyst... (2) adsorption of reactant molecules on surface of catalyst by forming loose bonds with catalyst due to free valencies... (3)occurence of chemical reactions between reactant and catalyst forming an intermediate.. (4)desorption of product molecules from surface due to its lack of affinity for the catalyst surface thereby making the surface free for fresh adsorption of reactant molecules... (5)diffusion of product molecules away from surface of catalyst...
- Q:A substance involved in chemical reflection, but reflects the quality of the material before and after the change, you say it is a catalyst?
- Is a catalyst. The catalyst is actually involved in the reaction, (these are multi-step reaction), but in the final time from the reaction out.
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Sodium Dichloroisocyanurate SDIC For Water Treament
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 9000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products





























