SDIC Aqua Products for Water Treatment Chemicals
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 1800 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
SDIC
Introduction:
CNBM--SDIC White powder or grain with chlorine odor . It is a strong oxidant and chlorate agent and can dissolved in water easily . Its aqueous solution assumes weak acidity and the active chlorine in its dry products lose little when it is stored for a long time at the atmospheric temperature .
SDIC Image:

SDIC Specification:
Chemical Name | Sodium Dichloroisocyanurate | |
Molecular Formula: | C3O3N3HCL2NA | |
Molecular Weight: | 220.96 | |
CAS Number: | 2893-78-9 | |
Product | 60% | 56% |
Available chlorine(%,min) | 60 | 56 |
Moisture content(% max) | 5 | 8 |
PH Value(1% solution) | 6-7 | 6-7 |
Particles Size:
Mesh | 5~8 | 8~30 | 20~40 | 20~60 |
Main usage:
this products can effectively kill various germs, fung uses and viruses, specially A&B type hepatitis viruses. It is effective on killing algae, decolorizing cleaning water or bleaching .It can be widely used for epidemic prevention, livestock farming , industry and agriculture.
Package:
50KG PLASTIC DRUMS/ FIBER DRUMS.
25KG PLASTIC DRUMS/FIBER DRUMS.
1000KG BIG BAGS.
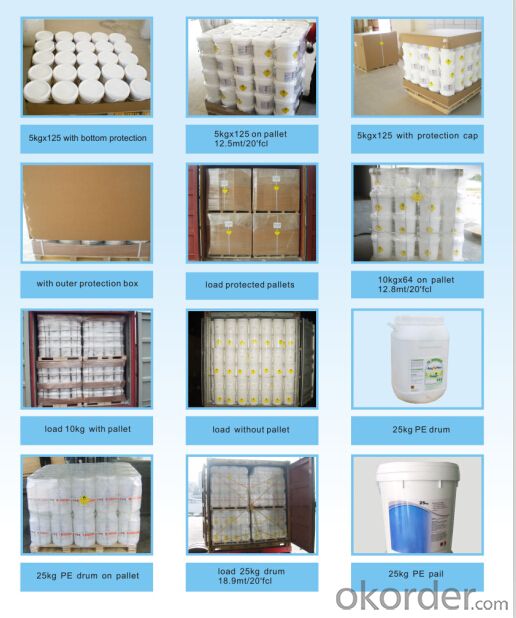
Or any other packages suggest by customers.
Service we can provide:
1. Mixed container, we can mix different items in one container.
2. Quality control, before shipment, free sample for test. after shipment, keep sample for 3 years
3. Prompt shipment with professional documents
4. Packing as your request, with photo before shipment
Our Advantages:
Full experience of large numbers containers loading in Chinese sea port
Fast shipment by reputed shipping line
Packing with pallet as buyer's special request
Best service after shipment with e mail
Cargoes together with container sales seervice available
Full experience for Canada & Japan export
Cargoes photo before and after loading into container
Raw materials from chinese origin
Professional Loading:
1.We will provide you with professional loading
2.We have one team supervise uploading the materials. We will check the container, the packages
3.Every step, taking pictures and make records.
4.we will make a complete Loading Report for our customer of each shipment.
- Q:Have you Read it ? If SoCan You Think Of Any Good Group Discussion Questions ?
- no but aOMG i laurie halse anderson! OMG I LOVED HER BOOK FEVER 1793 wooooooooooo that book was aweomse. you should read it
- Q:Brief introduction of enzyme as biocatalyst and general chemical catalyst and its personality
- can only change the rate of chemical reaction, do not change the equilibrium point of the chemical reaction, the enzyme itself does not change before and after the chemical reaction (3) can reduce the chemical reaction of the activation energy The
- Q:The best use of chemical catalysts
- Do not know what you want to use the best thing is what the catalyst is generally used in the process, there will be an initial induction period, the catalytic activity is relatively low, and then reached a stable catalytic state, this paragraph is generally called the catalyst life, the final Due to poisoning, active ingredient aggregation and so on factors, the catalyst activity will be reduced, then need to replace the new catalyst
- Q:Co and No form a chemical equation for Co2 and No2 under the action of a catalyst
- 2CO + 2NO == N2 + 2CO2
- Q:The chemical reaction equation of methanol heating and oxygen in the presence of catalyst
- Catalytic oxidation of formaldehyde
- Q:What chemical reactions can water do the catalyst?
- So far heard, but can and Na and other metal reaction
- Q:What are the chemical reaction conditions in organic chemistry are catalyst and heating, please elaborate
- This really does not have omnipotent law, their own more than one point, you can classify to remember, when I was in high school is in accordance with the notes, such as poly, polycondensation and the like. In general, the double triple bond addition, plus halogen is not the conditions, plus HCl, HBr and the like to heat; dehydration reaction generally concentrated H2SO4 heating, dehydration condensation is also; there are some special, such as ethylene added to ethanol Special temperature requirements, it seems that 120 degrees, there are other; other addition poly, polycondensation some need catalyst. The The In short, the conditions are many, in general, you do not go to the high school to do more questions after the feeling, encounter problems do not panic general experience can come out according to experience, this also depends on the usual accumulation, if the equation conditions Wrong to deduct points, it is not worthwhile. There are some questions when the examination will give you some information, whether you know do not know should see clearly, although some of the reaction but the subject to the conditions are not the same, when you do according to the title to write conditions, this will not wrong. In addition, thank you for your help, I do not seem to know you
- Q:What is the standard for the storage of flammable and explosive chemicals now?
- First, the basic requirements of classification of storage Dangerous goods, variety, complex performance, storage, in accordance with the zoning, classification, sub-section of the principle of special storage, set the number, set the number of fixed warehouses, fixed staff (four) custody. Small warehouses should be classified, divided, sub-stack storage, the performance of each other, fire fighting different items, dangerous dangerous goods and other general dangerous goods, should be stored separately.
- Q:Does all chemical reactions have a catalyst?
- Not some reaction without catalyst
- Q:In the catalyst and light conditions to break down the water to get the chemical equation of hydrogen
- 2H2O = (light or catalyst) 2H2 ↑ + O2 ↑
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
SDIC Aqua Products for Water Treatment Chemicals
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 1800 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Related keywords



























