Insulation Paper for Liquid Oxygen Storage
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 1 m²
- Supply Capability:
- 100000 m²/month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
The fire retardation glass fiber Cryogenic & Insulation paper is made of superfine glass fiber with special processing. This is usually used with aluminium foil and used for transport containers which store liquid helium, liquid hydrogen, liquid nitrogen,LNG etc.
The superfine glass fiber Cryogenic & Insulation paper's diameter between 0.1-0.6μm and fiber glass filament (diameter between 3-5 μm). According to a certain proportion, the paper is produced under the wet & vacuum forming process to make the paper have the space lattice structure. All the glass fiber is inorganic material, so it has outstanding flame retardant properties. Specifically engineered for use at big temperature range (approaching -273℃—500℃) and also the paper is really stable in the low temperature condition.
Cryogenic Insulation Paper Features:
The main way of heat conduction is by radiation heat exchange in the low and vacuum condition. In order to reduce the radiation heat exchange to meet the good heat insulation effect, by using aluminum foil for reflecting screen and superfine glass fiber insulation paper for spacer, then the multilayer combination of aluminum foil reflective screen and cryogenic & insulation material can build up the efficient thermal barrier against heat transfer in insulated vacuum storage containers. This material can be freely winding, and help the user to reduce the waste of manpower and material waste.
Cryogenic Insulation Paper Application:
Cryogenic liquid (liquid helium, liquid hydrogen, LNG, liquid oxygen, liquid nitrogen, liquid argon, etc.)
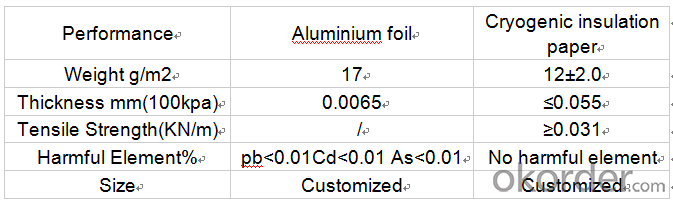
Cryogenic Insulation Paper Specifications
1.light weight
2.good uniformity
3.low thermal conductivity
Technical data:
Q1 What’s the transport method?
A1 FCL delivery goods with wooden pallet; If LCL delivery, must with wooden case; Sometimes need open top, flat rack or bulk cargo.
Q2 What’s the required payment term?
A2 Generally 30% TT as the prepayment, 70% TT before delivery. If need, 100% Irrevocable Letter of Credit or negotiation.
Q3 Which country are our products exported to?
A3 Apart from entire Chinese market, the US, Russia, Japan, Korea, Australia and some Southeast Asian Nations.


- Q:Where can I apply for medical electrical equipment training?
- According to the contents of the bulletin, the EU member states must be in June 7, 2000 to complete the implementation of the instructions required by the relevant laws and regulations ordered to amend the order and since December 2003, all members of the EU sales in vitro diagnosis Medical equipment (In Vitro Diagnostic Medical Devices,
- Q:Which ultra-low temperature refrigeration equipment trust? Professional to do this cold chain, refrigeration equipment this one, scientific research needs.
- Made this piece of Aucma, Haier have to do. Imports like Sanyo's, Sanyo, but now also made. Yes, there are thermoelectrics.
- Q:Medical disposable surgical kits belong to several types of medical device equipment and numbered codes
- One-time use of surgical bags belong to the second category 6864 (No.) medical hygiene materials and dressings, the scope of use is: for medical institutions to use the patient surgery.
- Q:Domestic manufacturers of medical oxygen equipment? Quality must be better, the best brand, seeking recommendations.
- Do the oxygen equipment manufacturers are not many, there are many installation and construction, can really have a specific construction program and the process of the enterprise is very small.
- Q:Need to do a low-temperature equipment, the temperature inside the box to minus 70 degrees, the insulation layer of 100 mm, what is the insulation layer of material for good insulation? Check with polyurethane PU foam is better, but more expensive. With glass fiber cotton insulation afraid of a long time the insulation layer will have condensate and damage the insulation effect, but look at other foreign similar equipment are used glass fiber cotton insulation, may I ask, in the end can use this material to low temperature insulation? There are detailed technical information that I add another 100 points, thank you!
- Your equipment temperature is zero, you should find cold material! Polyurethane is insulation material! Do not mix the concept!
- Q:Will the principle and function of medical water equipment? Medical water equipment to how much money?
- The biggest advantage of using reverse osmosis water purifier is that the water filter is very clean, than the outside to sell the mineral water but also clean a lot, the biggest drawback is the small amount of water can not be used as family life to water, and the higher cost.
- Q:Will the degree of zero is ultra-low temperature, cold storage is a special equipment?
- Pressure vessel, refers to the gas or liquid, carrying a certain pressure of the closed equipment, the scope of the provisions of the maximum working pressure greater than or equal to 0.1MPa (gauge pressure) of the gas, liquefied gas and the maximum operating temperature is higher than or equal to the standard boiling point of the liquid , A fixed container and a movable container having a volume greater than or equal to 30 L and an inner diameter (non-circular cross-section refers to a maximum geometric dimension within the cross-sectional boundary) greater than or equal to 150 mm; a nominal operating pressure greater than or equal to 0.2 MPa (gauge) The product of pressure and volume is greater than or equal to 1.0MPa ? L of gas, liquefied gas and standard boiling point is equal to or below 60 ℃ liquid cylinders;
- Q:Refinery low-temperature equipment which, high-temperature equipment which?
- This problem is not good to answer, with a different device in different devices may become a low-temperature equipment, may become a high-temperature equipment
- Q:Henan Kaifeng in 2015 for the examination of medical equipment, when the job certificate to receive
- Receive the original ID card (military officer with a military card), can be on behalf of the collar, who is required to carry the original identity card.
- Q:Medical low temperature refrigerator offer is how much
- Medical low temperature refrigerator Price: ① style: vertical or horizontal, generally vertical than the horizontal price;
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Insulation Paper for Liquid Oxygen Storage
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 1 m²
- Supply Capability:
- 100000 m²/month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Related keywords