Extruded Aluminum Panel For Roofing Building Application
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 60000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Item specifice
Extruded Aluminum Panel For Roofing Building Application
Specifications
Alloy NO. | Thickness | Width | Length | Production line | Circulation size |
1050 1060 1100 3003 | 0.3-9.5mm | 800--2200mm | 1000--10000mm | DC,CC | 1220*2440mm 1250*2500mm 1500*3000mm 1000*2000mm
|
5052 5083 5454 5754 5086 | 0.5-150mm | 800--2200mm | 1000--6000mm | DC | |
6061 | 5-150mm | 800--2200mm | 1000--10000mm | DC | |
7075 | 6-150mm | 800--2200mm | 1000--10000mm | DC | |
Packing | Stick blue film→plastic film→waterproof paper→1~2 tons on a export standard pallet(corner protection) | ||||
Application | decoration:ceilings,walls,furniture,cabinets,elevators,signs,name plate, transportation, cookware, household appliances:refrigerators,microwave ovens, machinery, mold making,aerospace and military aspects, auto, PP cap , construction etc | ||||
Standard | Chemical Composite: GB/T 3190-2008, JIS, EN, ASTM | ||||
Mechanical Property: GB/T 3880.2-2012, JIS, EN, ASTM | |||||
MOQ | 5TONS | ||||
Kind attention | Specifications can be customized as the customer’s requirements | ||||
Application

Shipment
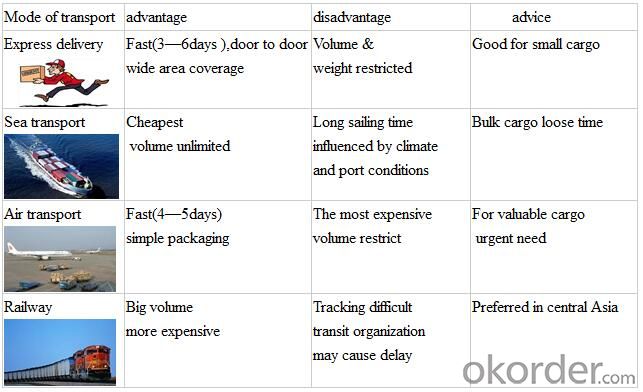
FAQ
Q: Can you provide free samples?
A: Yes, free samples will be sent to you on freight at destination.
Q: Can I get your latest catalogue?
A: Yes, it will be sent to you in no time.
Q: What are your payment terms?
A: We accept L/C, D/A, D/P, T/T, West Union, etc
Q: Can you provide free samples?
A: Yes, free samples will be sent to you on freight at destination.
Q: Can I get your latest catalogue?
A: Yes, it will be sent to you in no time.
Q: What is the MOQ?
A: 5 tons.
- Q:i've been looking at stove top espresso coffee makers and they are all made of either aluminum or stainless steel. so i wonder is one metal any better than the other as far as coffee makers go? i thought before i actually buy one i'd like to hear from you - do you have a stove top espresso maker? what metal is it and what do you think of it? looking forward to reading your thoughts - thanks everyone!cheers!
- For general cookware both metals have different attributes. Aluminum conducts heat better and therefore pots and pans heat up more evenly. Stainless steel on the other hand has a nicer look and doesn't discolor and oxidize the way aluminum might, and it cleans up better. For an espresso maker you really don't need even heating since you are only heating water in a really small area. Therefore you should go with stainless steel.
- Q:Are 101 aluminum sheets suitable for marine environments?
- Yes, 101 aluminum sheets are suitable for marine environments. 101 aluminum is a high-strength alloy that is known for its excellent corrosion resistance, making it ideal for use in marine applications. It can withstand the harsh conditions of saltwater, including exposure to salt spray and water immersion, without corroding or deteriorating. Additionally, 101 aluminum has good weldability and formability, allowing it to be easily fabricated into various marine components such as boat hulls, decks, and equipment. Its strength, durability, and resistance to corrosion make 101 aluminum sheets a reliable choice for marine environments.
- Q:Other then pop cans, what else is aluminum that can be recycled?
- Anything made of aluminum can be recycled. Aluminum cans are just a visible item that can be used for social engineering purposes. The cans also are very pure form of the metal. You will find aluminum in many things, from parts in your computer, major parts in your family car, pots, pans, kitchen items, tools, display items, structural supports for various things, boats, canoes, airplanes, and the list is long. Fact is, it would be difficult to find many items where aluminum was NOT used. Although everything that is metal that a magnet would not stick to, is not always aluminum. ANY metal can be recycled, and most metals can be sorted for recycling when you find a recycling plant that will buy the materials. Some of the metals need to be in large quantity, or weight in order to be worthwhile sorting. Some things like batteries can be sorted by general type. Some of those are easier to break down to component metals than others. Some have hazardous substances in them so need care in storage, and some have hazardous substances that need special care in shipping them. But they are exception.
- Q:Can aluminum sheets be soldered?
- Yes, aluminum sheets can be soldered, but it requires some specific techniques and materials. Aluminum has a high thermal conductivity and a low melting point, which makes it challenging to solder using traditional methods. However, it can be soldered using specialized soldering techniques such as the use of flux and a soldering iron with a high temperature. Additionally, a specific type of solder called aluminum solder or aluminum flux-cored solder is required, as regular solder does not adhere well to aluminum surfaces. It is important to clean the aluminum surface thoroughly before soldering to ensure a strong bond. Overall, while soldering aluminum sheets can be more complex compared to other metals, it is possible with the right tools, materials, and techniques.
- Q:Can 101 aluminum sheets be bent or formed into different shapes?
- Certainly! It is indeed possible to bend or shape 101 aluminum sheets into various forms. Aluminum possesses high malleability, meaning it can be shaped effortlessly without any risk of breaking or cracking. The specific grade of aluminum, such as 101, signifies the alloy composition and might possess specific properties that make it more suitable for bending and shaping. Nevertheless, the capacity to bend or shape aluminum sheets also relies on their thickness; thicker sheets may necessitate greater force and specialized equipment. In conclusion, with the correct tools and techniques, it is feasible to bend or shape 101 aluminum sheets into diverse shapes to fulfill specific requirements.
- Q:Are aluminum sheets suitable for food storage containers?
- Yes, aluminum sheets are suitable for food storage containers. Aluminum is a lightweight and versatile metal that is commonly used for food packaging and storage. It is non-toxic, non-reactive, and does not impart any taste or odor to the food stored in it. Aluminum containers are also resistant to corrosion, which helps to keep the food fresh and prevent any contamination. Additionally, aluminum is a good conductor of heat, allowing for efficient cooling or heating of the food. It is also recyclable, making it an eco-friendly choice for food storage containers. Overall, aluminum sheets are a popular and reliable option for storing food safely and effectively.
- Q:which has more volume? a kg of gold or a kg of aluminum? please explain in physics terms
- Think of it this way: Which metal is lighter? How do you know this? Because a given volume of gold weighs more than a given volume of aluminum. So which one has more volume? A kg of aluminum.
- Q:Can aluminum sheets be etched or engraved?
- Indeed, it is possible to etch or engrave aluminum sheets. The process entails removing a portion of the material from the surface by utilizing acid or a laser with substantial power. This technique allows for the creation of detailed designs, patterns, or even textual elements on the aluminum sheet. The applications for etching or engraving are diverse, including signage, decorative items, and industrial components. To achieve the desired appearance, color or other finishes can be added to further enhance the resulting design on the aluminum sheet.
- Q:What are the benefits of using aluminum sheets?
- There are several benefits of using aluminum sheets in various applications. Firstly, aluminum sheets are lightweight, making them easy to handle and transport. This characteristic is particularly advantageous in industries such as aerospace, automotive, and construction, where weight reduction is crucial for fuel efficiency and overall performance. Secondly, aluminum sheets offer excellent corrosion resistance. Unlike other metals, aluminum naturally forms a protective oxide layer when exposed to air, preventing further corrosion and enhancing its durability. This makes aluminum sheets highly suitable for outdoor applications or in environments with high humidity or corrosive substances. Another benefit of using aluminum sheets is their high strength-to-weight ratio. Aluminum is known for its exceptional strength, allowing it to withstand heavy loads or extreme conditions without compromising its structural integrity. This makes it an ideal choice for constructing lightweight but sturdy structures, such as building facades, vehicles, or industrial equipment. Additionally, aluminum sheets are highly malleable and ductile, meaning they can be easily formed into various shapes and sizes. This flexibility allows for intricate designs and customization, making aluminum sheets highly versatile and adaptable to different manufacturing processes or project requirements. Furthermore, aluminum is a sustainable and environmentally friendly material. It is 100% recyclable, meaning it can be reused repeatedly without losing its properties or quality. Recycling aluminum requires significantly less energy compared to the production of primary aluminum, resulting in reduced carbon emissions and a reduced environmental impact. Lastly, aluminum sheets have excellent thermal and electrical conductivity properties. This makes them suitable for applications that require efficient heat transfer or electrical conductivity, such as heat exchangers, solar panels, or electrical wires. Overall, the benefits of using aluminum sheets include their lightweight nature, corrosion resistance, high strength-to-weight ratio, malleability, sustainability, and excellent thermal and electrical conductivity. These advantages make aluminum sheets a popular choice in various industries, contributing to improved performance, cost-effectiveness, and environmental sustainability.
- Q:Can the aluminum sheets be used in food or beverage processing industries?
- Yes, aluminum sheets can be used in food or beverage processing industries. Aluminum is a commonly used material in these industries due to its excellent properties such as corrosion resistance, lightweight, and ability to withstand extreme temperatures. It is often used in food packaging, processing equipment, and beverage cans.
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Extruded Aluminum Panel For Roofing Building Application
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 60000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Related keywords




























