drinking water chlorine tablets TCCA ISO Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 22 m.t
- Supply Capability:
- 1800 m.t/month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
TCCA 90% Powder, Granular, Tablets
Introduction:
CNBM –TCCA White tablet with irritant chlorine odor. Slight solute in water. TCCA is the organic compound with the formula (C3Cl3N3O3). It is used as an industrial disinfectant, bleaching agent and a reagent in organic synthesis. This white crystalline powder, which has a strong "chlorine odour," is sometimes sold in tablet or granule form for domestic and industrial.
Specification:
Chemical Name | Trichloroisocyanuric Acid |
Molecular Formula | CONCL3 |
CAS Number | 87-90-1 |
Avaliable Chlorine %min | 90.00 |
Moisture content %,wt,Max | 0.30 |
PH Value (1% solution) | 2.7~3.3 |
Solubility 25℃ Water | 1.2g/100g |
Solubility 30℃ Acetone | 36g/100g |
Specific Gravity | 0.95(light)/1.20 |
Granular Particles Size
Mesh | 5~8 | 8~30 | 20~40 | 20~60 |
Tablets Forms
Weight | 200 gram | 150gram | 100gram | 50gram | 30gram | 20gram | 15gram | 10gram |
Diameter(mm) | 76 | 70 | 50 | 42 | 30 | 30 | 30 | 30 |
Height(mm) | 25 | 21 | 26 | 27 | 22 | 16 | 12 | 8 |
Multi-Functions
We made multifunctional tablets according to customers’ needs. For Instance, we press TCCA 90% granular with chemicals such as Boric Acid,. Sulfate Copper, Sulfate Aluminum And PAC.
Packing:
Granular& Powder:
50KG PLASTIC DRUMS/ FIBER DRUMS.
25KG PLASTIC DRUMS/FIBER DRUMS.
1000KG BIG BAGS.
Or any other packages suggest by customers.
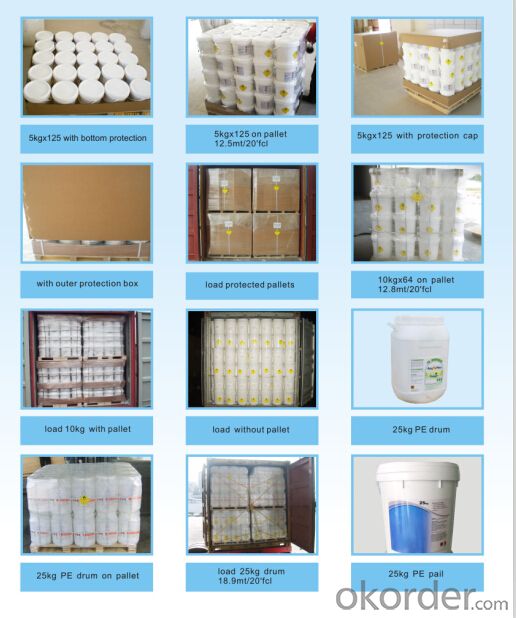
Tablets:
Inner Packing:
Individually Wrapped for 200gram,150gram, 100gram
1kg Plastic tube for 200gram
1kg plastic bottle & 5kg plastic bottle for smaller tablets
Outer Packing:
50KG,25KG,10KG, 5KG plastic Drums.
Or any other package specified by customers, such as fiber drums or cantons.
- Q:Why the catalyst after the chemical reaction of its quality and chemical properties unchanged
- In fact, the catalyst in the reaction process has become other substances, but after the end of the reaction, the catalyst has changed back. That is, the catalyst is actually involved in the reaction, except that the amount of catalyst being reacted is as much as it did.
- Q:Effect of Catalyst on Chemical Reaction Rate
- The catalyst can reduce the activation energy of the chemical reaction and allow the reaction to take a shortcut
- Q:What is the nature of the chemical catalyst?
- Different reactions have different catalysts, mainly catalyzed, to speed up the reaction rate
- Q:What are the characteristics of the catalyst in the catalytic reaction?
- The role of the catalyst in the chemical reaction is to change the rate of chemical reaction, and its own quality and chemical properties do not change.
- Q:What kind of chemical substances can seriously damage the ozone layer, as a catalyst or reactants can be?
- Freon decomposition of free radicals. The destruction of our ozone is mainly caused by him. Chlorine free radicals are also available.
- Q:The catalyst before and after the reaction of the quality and nature of the same, does it mean that a little catalyst can be all the substrate reaction? Such as: one gram of MnO2 can reflect the infinite H2O2?
- The quality of the catalyst before and after the reaction
- Q:Is the catalyst used in the starch phosphate reaction
- (Cat1, cat2, cat4 and cat5) in the presence of terephthalic acid,
- Q:how do catalysts help in green chemistry?
- Catalysts reduce the energy barrier for reactions, meaning they require less energy to make the forward reaction go. This means less heat, light, or other energy sources are required to perform the same reaction without a catalyst. Also, by definition, a catalyst is not consumed in a reaction and can therefore be recycled many many times before replacement is necessary.
- Q:My chemistry teacher wont tell me because it's in the higher course. And i'm not waiting a whole year to find out. And also, google is being a gimp about it. So thanks a lot if you know, I only have basic chemistry knowledge btw, lumen'ss terms if you can.
- MAD = Mutual certain Destruction you could injury us, of direction. yet you could no longer injury us till now we, even from the grave injury you too. we can be lifeless, we understand that. yet you will no longer stay to confirm and revel in it. what would be left of the two one human beings will ought to combat the subsequent war with sticks and stones. 3 skill of dropping the bomb: by skill of airplane, by skill of submarine launch and by skill of ICBM Inter Continental Ballistic Missile. As the two events found out war exchange into certain destruction, neither fairly needed that. We have been given fairly near to nuclear war, exceedingly throughout the Cuba disaster. yet provided that a nuclear war might propose the certain finished destruction of and the U.S. and the u . s . and West + East Europe, it must be prevented. in assessment to on the instant. have been non secular fans already have the bomb (Pakistan) or very near to construct one (Iran) and don't care a rattling concerning the outcomes. Allah will supply! (them with seventy two virgins each and each)
- Q:What are the pharmaceutical manufacturing companies now using PT / AL_203 catalysts?
- Yueyang Eagle Hill Petrochemical Plant
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
drinking water chlorine tablets TCCA ISO Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 22 m.t
- Supply Capability:
- 1800 m.t/month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Hot Searches
Related keywords




























