Cyanuric Acid Lead Granular Tablets Powder Standrd Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 1800 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Cyanuric Acid
The Structure of Cyanuric Acid Descriptions:
Trade Name: Isocyanuric Acid
Other name: Cyanuric Acid; 1,3,5-Triazine-2,4,6-triol
Uses: Bleaches and sanitisers.
Formula: C3H3N3O3
Molecular Weight: 129.07
CAS NO.: 108-80-7
Main Features of Cyanuric Acid:
White powder, granular or colored tablet form, non-toxic and odorless
Full experience of large numbers containers loading in Chinese sea port
Fast shipment by reputed shipping line
Packing with pallet as buyer's special request
Best service after shipment with e mail
Cargoes together with container sales seervice available
Full experience for Canada & Japan export
Cargoes photo before and after loading into container
Raw materials from chinese origin
Cyanuric Acid Specification:
| ITEM | SPECIFICATION | RESULT | |||
| Content | ≥98.5% | 98.64% | |||
| Moisture | ≤0.5% | 0.11% | |||
| PH value | 4.0-4.5 | 4.26 | |||
| Fe2+ | ≤15ppm | 7.5ppm | |||
| NH4+ | ≤200ppm | 97ppm | |||
| Ash | ≤0.1% | 0.05% | |||
| Insoluble matter in DMF | ≤0.3% | 0.25% | |||
| Appearance | White crystalline power | White crystalline power | |||
| Mesh number | 95% pass 80 mesh | 95% pass 80 mesh | |||
| White degree | ≥89 | 90.5 | |||
| Conclusion: | The product complies with the standard above. | ||||
Cyanuric Acid Packing:
in 25kg, 1000kg bag for powder
in 25kg plastic bag or 50kg PE drums for granular

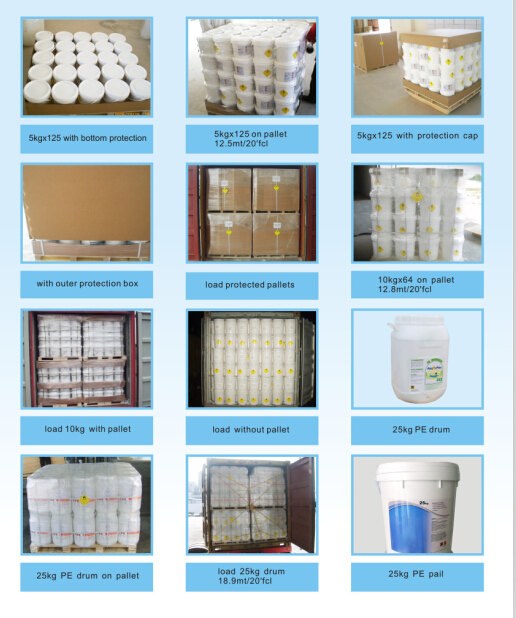

Service we can provide:
1. Mixed container, we can mix different items in one container.
2. Quality control, before shipment, free sample for test. after shipment, keep sample for 3 years
3. Prompt shipment with professional documents
4. Packing as your request, with photo before shipment
Professional Loading:
1.We will provide you with professional loading
2.We have one team supervise uploading the materials. We will check the container, the packages
3.Every step, taking pictures and make records.
4.we will make a complete Loading Report for our customer of each shipment
- Q:Why the amount of catalyst is too small will make the chemical reaction rate slowed down
- Whether the chemical reaction can be carried out according to the change of free energy, but only according to the change of free energy can not determine whether the reaction can be completed, because the chemical reaction is also completed by the reaction of the energy barrier, that is, if the reaction energy barrier is high, To provide some energy, across the barrier, to complete the reaction. The energy barrier is called activation energy. And the role of the catalyst is to reduce the activation energy, so that in a relatively harsh environment, chemical reaction occurs.
- Q:Is the reaction of the exhaust purification of cars (carbon monoxide and nitrogen
- 2NO2 + 4CO = catalyst = N2 + 4CO2
- Q:What is the difference between biological and chemical catalysts?
- Biological catalyst: 1. Biological catalysts or enzymes are high molecular weight globular proteins. 2.Their composition may change at the end of reaction. 3.Their catalyzing effect is very high. i.e faster than chemical catalyst. 4.They are reaction specific. i.e One enzyme or biological catalyst may catalyze only particular type of reaction and not many. 5.They are intolerant to temperature and pH changes. An enzyme can not function outside its temperature or pH range. e.g amylase,lipase,pepsin Chemical catalyst: 1.Chemical catalysts are simple inorganic molecules with low molecular weight. 2.They remain unchanged at the end of reaction. 3.They are slower compared to enzymes. 4.They are not reaction specific. 5.They function within wide range of temperatures,pH or pressure. e.g vanadium dioxide, platinum
- Q:Brief introduction of enzyme as biocatalyst and general chemical catalyst and its personality
- enzyme inactivation (4) enzyme activity can be timely and effective regulation (5) the role of the enzyme conditions are more moderate (6) most of the enzyme (1) the catalytic efficiency is very high (2) Of the catalytic activity is often associated with coenzymes, auxiliary or metal ions, and some enzyme activity also need RNA as a cofactor Caixing, such as telomerase
- Q:Chemical catalyst system baa?
- Catalyst is divided into inorganic catalyst and organic catalyst organic reaction generally need more catalyst, such as concentrated sulfuric acid
- Q:Why can't catalysts make an unfavorable reaction favorable?Can anyone give me a relatively simple explanation for this?Thank you so much in advance!
- Catalysts, as enzymes, only change the activation energy (the energy the compound needs to gain to transform into products), they don't change the Gibbs energy values of reactants nor products. Therefore, if the delta G of the reaction is positive, it'll still need free energy to complete. They make a reaction complete faster than in normal conditions, but don't change the actual possibility for that reaction to happen. In the human body, a lot of reactions of catabolism have a positive G value and these reactions needs to get energy from other coupled reactions that have a negative value, so the total value is still negative. Many of them use hydrolysis of ATP to provide that energy, as its hydrolysis is about -30 kJ/mol in physiological conditions. I don't know what class you're in to ask this question, so can't really know if this answer is too simple or complicated for u... sorry in advance! Jo?l
- Q:Does a catalyst work for both reactants and products?From my understanding, organic catalyst or enzyme does not necessarily work for the product of the reaction because of the shape of the activation site. However I cannot speak for inorganic ones.
- A catalyst works for either the reactants or the product in a given reaction. If it works for the reactants then the activation site on the enzyme, for example, will only fit on the reactants. If a catalyst is added to a reaction in which it catalyzes the back-reaction, or it aids the products, then the reaction will be reversed. i don't think a catalyst could work for both the reactants and products in one reaction because it wouldn't really be a catalyst anymore; it wouldn't make the rxn progress any faster since it would be canceling out itself by aiding both the reactants and the products. I think this is what you are asking, if not please post more details. I hope I didn't confuse you even more!
- Q:When you write a chemical equation, how do you want to add "catalyst" and "?" When you do not have to write?
- This is the need for your memory, write a few times, will naturally cooked
- Q:Hydrogen and oxygen in the role of the catalyst can do the chemical formula of aviation fuel?
- You two yeah? Meaning that hydrogen is ignited in oxygen to release energy to push the rocket forward, as the catalyst for those who love,
- Q:What is the difference between an enzyme catalyst in a living body and a catalyst in chemistry?
- Enzyme has a high catalytic efficiency (high efficiency) Generally speaking, the reaction rate of the alcohol catalyzed reaction rate is 106-1013 times higher than that catalyzed by the chemical catalyst
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Cyanuric Acid Lead Granular Tablets Powder Standrd Quality
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 17 m.t.
- Supply Capability:
- 1800 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Related keywords



























