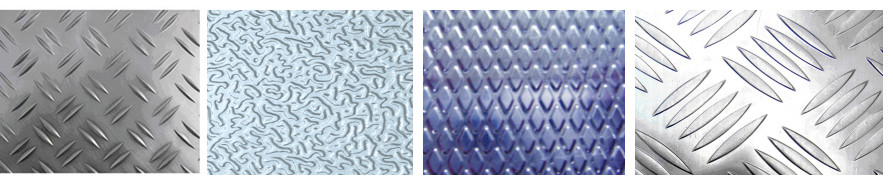
Anti Slippery Aluminum Sheet, Plate,Stucco
- Loading Port:
- Shanghai
- Payment Terms:
- TT or LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 9000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Textured Aluminum Sheet Tread Plate , Five Bars Embossed Aluminum Sheet
Aluminum Plate, Aluminum Sheet, Aluminum Roll, Aluminum Coil, Aluminum Alloy

Product | Textured Aluminum Sheet Tread Plate , Five Bars Embossed Aluminum Sheet
|
Material | 3003(3000 series) |
Status | H14 |
Thickness | 0.5-2.0mm |
Aluminum content | ≥ 95.00% |
Surface | mill finish,no scratching,without burrs |
Property | good extensility,anti-corrosion,good welding performance |
Technical standard | GB/T3880-2006 |
Brand name | CNBM |

Division | Alloy | Application | Feature |
1000 SERIES | 1050 1060 1070 1200 | Utensil, decoration, Reflecting plate, Printing plate, heatproof plate | -Easy to process and weld, resistant to rust, High conductibility of electricity and heat, low strength -Kinds of Cookware |
3000 SERIES | 3003 3005 3105 | Utensil (F/P, inside of rice cooker), aluminum can, material for interior and exterior of building, Celluar Phone | -A3003 : Anodizing Cookwares, general wares, frying pan, coating, Pressure cooker, electric rice cooker, Utensil, Mobile Phone cover & Battery Case Cheaper item with standard quality |
5000 SERIES | 5083 5052 5754 5A05 | Ship board heatproof apparatus, material for interior and exterior of building, Parts of Electronic tools Automobile Components: | Easy to process and weld and superior hardness &heatproof |
6000 SERIES | 6061 6063 | IT equipment & facility, Mould material, motor material, automatic line, machine & plant etc. | -Easy to process , resistant to rust, superior surface treatment |
8000 SERIES | 8011 | Kitchen utensils, Bottle cap | -8000 series is durable to process with special alloy. |

- Q:Is the chemical formula for Aluminum Sulfate Al2(SO4)3?How and why is Al2(SO4)3 wrong?
- Formula For Aluminum Sulfate
- Q:i've been looking at stove top espresso coffee makers and they are all made of either aluminum or stainless steel. so i wonder is one metal any better than the other as far as coffee makers go? i thought before i actually buy one i'd like to hear from you - do you have a stove top espresso maker? what metal is it and what do you think of it? looking forward to reading your thoughts - thanks everyone!cheers!
- stainless steel is heavier and holds onto heat longer its also non-reactive aluminum can react to acids, changing flavors, and coffee has acids, so this could affect the taste.
- Q:Does anyone know why Mercury -(Thimerosal) is used in Flu Vaccinations and where Mercuryisnot used Aluminium in other vaccines ? While these are known to be toxic metals, can these cause headaches, severe hot burning heads and or Sinusitus ?
- Toxicity is about dose. The dose of mercury in the flu vaccine is minute and cannot possibly contribute to mercury poisoning. In any case, Thimerosal converts to ethylmercury in the body which is quickly broken down and excreted - unlike methylmercury which is the mercury you are thinking about. A tuna fish sandwich will give you more mercury than any vaccine. Thimerosal is a preservative basically, which is why it is added to the multi-dose vial. If you are that paranoid about it, ask for the single dose vial to be used.
- Q:Is aluminum sheet recyclable?
- Yes, aluminum sheet is highly recyclable. Aluminum is one of the most sustainable and environmentally friendly materials, as it can be recycled indefinitely without losing its quality. The recycling process of aluminum sheet involves melting it down to its liquid form, which requires significantly less energy compared to the production of primary aluminum from raw materials. Recycling aluminum sheet not only conserves natural resources but also reduces greenhouse gas emissions and saves energy. It is estimated that recycling one ton of aluminum sheet saves about nine tons of CO2 emissions. Therefore, aluminum sheet recycling is not only economically viable but also an essential practice for maintaining a sustainable and eco-friendly environment.
- Q:I was hoping someone could explain aluminum corrosion with degreasers and cleaning formulas for automotive cleaning. This starts with, where is this aluminum (wheels)?, what types of cleaners?, do cleaners cause corrosion? Any insights would be appreciated.
- Contrary to popular believe, aluminum oxidizes just like steel. Steel rusts to a reddish color. Aluminum rusts to a white color and looks more like corrosion. Aluminum cleaners or cleaners safe for aluminum usually clean without staining the aluminum surface. Hardly any cleaner for for automotive use will corrode aluminum. Visit your local auto parts store and read the labels for a wheel cleaner that is 'safe' for aluminum. You should be able to use it on all aluminum surfaces.
- Q:My professor gave us the following:1roll of Reynonlds Wrap Heavy Duty Aluminum18.0 in wide, 500.0 ft long and 0.94mil thickThe cost $48.98and I'm not sure how to figure it out
- length * width * thickness = volume (with some unit conversions) Mass = volume * density (look up the density of aluminum) convert mass to moles. convert moles to atoms Divide the cost by the number of Al atoms.
- Q:Can aluminum sheets be used for packaging purposes?
- Aluminum sheets have the ability to serve as packaging material. Due to its exceptional barrier properties, durability, and lightweight nature, aluminum is a versatile and favored choice for packaging. It is commonly employed in various forms, such as aluminum foil, cans, and containers, to pack a diverse range of products like food, beverages, pharmaceuticals, and cosmetics. The utilization of aluminum sheets for packaging offers numerous advantages. These sheets possess resistance against moisture, oxygen, light, and bacteria, which ensures the protection and preservation of the packaged contents. Furthermore, aluminum sheets can be easily manipulated, shaped, or folded to create a variety of packaging designs, making it a preferred option for numerous packaging applications.
- Q:How do you prevent fingerprints on aluminum sheets?
- There are several strategies you can use to prevent fingerprints on aluminum sheets: 1. Opt for gloves: To avoid leaving fingerprints on aluminum sheets, a simple solution is to wear gloves while handling them. This creates a barrier between your hands and the surface, preventing any marks or smudges. 2. Thoroughly clean the surface: Before touching aluminum sheets, ensure that the surface is clean. Use a suitable cleaning agent or mild detergent to remove any dirt or oils. Dry the sheets completely with a lint-free cloth to minimize the chances of fingerprints sticking to the surface. 3. Apply a protective coating: Prevent fingerprints by applying a protective coating on the aluminum sheets. Clear lacquer or a clear protective film designed for this purpose are viable options. These coatings create a barrier that reduces the visibility of fingerprints and makes them easier to clean off. 4. Handle with care: Minimize direct contact with aluminum sheets to reduce the likelihood of fingerprints. Fingerprints are more likely to occur when pressure is applied or when the surface is repeatedly touched. Whenever possible, use tools or gloves to manipulate the sheets instead. 5. Proper storage: Ensure that aluminum sheets are stored in a clean and dust-free environment, away from potential contaminants. Consider using protective sleeves or covers to shield the sheets from fingerprints and other marks. By implementing these preventive measures, you can significantly decrease the occurrence of fingerprints on aluminum sheets, maintaining their cleanliness and visual appeal.
- Q:the aluminium pull tabs are in a jucy juice bottle
- So saving pull tabs isn't a complete waste of time. But let's make one thing clear: *there's nothing special about pull tabs*. You'd save yourself a heap o' trouble and make a lot more money if you recycled the whole can. The Reynolds and kidney foundation people have tried to get that point across with a poster showing a red Ghost busters-type slash through a cartoon of someone trying to detach a pull tab from a can. The headline says, Keep Tabs on Your Cans. But the public hasn't gotten the message. Supposedly responsible people -- e.g., the honchos at your school -- will organize pull tab collection drives without even bothering to get the whole story. Urban legends expert Jan Brunvand reports that in 1989 a Minneapolis VFW post organized a pull tab collection drive for the local Ronald McDonald House. When Brunvand asked the organizers why they didn't tell people to save whole cans, they lamely replied that there were hygiene problems and that people liked mailing in the tabs, even though the postage often exceeded the value of the aluminum. In other words, it's not important to *do* good as long as people *feel* good. Sometimes I don't think we have enough common sense in this country to fill a teacup.
- Q:I constructed a small hho generator for experimental purposes. I needed to add a cooling condenser to the unit because of a overheating problem. I was using a old heater core from a vehicle, soldered some joints to make some connections. the solution seemed to have eaten the solder I used, which was normal lead free solder used in water pipes. I was going to invest into a trans cooler core which is made of aluminum, but I need to know if the solution will eat up the aluminum. I also thought about using a condenser core from a broken window a/c, which is made of copper tubing. any advise would be very help full
- Figure out the Eknots using a chart. Cu -- Cu2+ = Enot of ? Al3+ --- Al = Enot of ? It needs to be positive to spontaneously react. I believe from memory that Al -- Al3+ is 1.3volts, so the reverse is negative. Meanwhile Cu is something under .5 volts, so it won't be enough to make the reaction work. This is further understood because aluminum requires a lot of energy to smelt from the oxide (which is the reverse reaction of Al3+ -- Al that you're talking about here) Secondly, do your homework man, it's REALLY obnoxious to add the why or why not and phrase everything exactly as your homework asks. Try to ask a question about the topic so you actually learn. For example, what are reduction potentials in terms of, for example, Cu --- Cu2+
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Anti Slippery Aluminum Sheet, Plate,Stucco
- Loading Port:
- Shanghai
- Payment Terms:
- TT or LC
- Min Order Qty:
- 5 m.t.
- Supply Capability:
- 9000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Hot Searches
Related keywords






























