Amino Trimethylene Phosphonic Acid Liquid And Solid
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 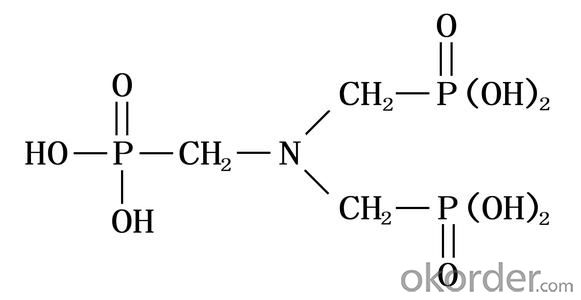
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q:Is the reaction of the exhaust purification of cars (carbon monoxide and nitrogen
- NO + 2CO = 2CO2 + N2 reaction is exothermic. Conditional catalyst
- Q:What is the PTC catalyst in chemistry?
- 1, polyether chain polyethylene glycol: H (OCH2CH2) nOH chain polyethylene glycol dialkyl ether: R (OCH2CH2) nOR2, cyclic crown ethers: 18 crown 6,15 crown 5, Fine and so on. 3, quaternary ammonium salt: commonly used quaternary ammonium salt phase transfer catalyst is benzyl triethyl ammonium chloride (TEBA), tetrabutyl ammonium bromide, tetrabutylammonium chloride, tetrabutylammonium hydrogen sulfate (TBAB) , Trioctylmethylammonium chloride, dodecyltrimethylammonium chloride, tetradecyltrimethylammonium chloride, and the like. 4, tertiary amine: R4N X, pyridine, tributylamine and the like. 5, quaternary ammonium base (its alkaline and sodium hydroxide similar) soluble in water, strong hygroscopicity. 6, quaternary phosphonium
- Q:how could scientists know the exact catalyst for every reactions??? THANX sooo much
- Believe me, nema, there's no way that we chemists know the best catalyst for every reaction. That would be simply impossible. However, from the type of reaction, the reactants, products, reaction conditions, solvents, etc. and from one's experience and the literature (papers and patents) one can get a good idea for most reactions of the type of catalyst that has worked for similar systems. One then starts off with a catalyst from the literature and modifies or changes it if improvement is needed based on chemical principles that one learns. There are also some theoretical calculations that can be made. Sometimes they work and sometimes they don't :) If it is an industrially important process like the Haber process for making ammonia from nitrogen gas and hydrogen gas, there may be thousands of catalysts which have been tried and evaluated. New minor improvements are being made every day. When a company does find a very good catalyst for an important reaction, often they keep it a trade secret. The good catalyst can make a huge difference in how commercially successful a particular process is. That's a large part of what chemical engineers do. You may never know if you have the best catalyst. The most you can hope for is one that is good enough. So it's a few parts personal knowledge, a few parts literature, a couple of parts theory, a lot of experimentation and often, more than not, a little luck. :)
- Q:about 1-3 sentences on this will do thank you
- Catalysts (including enzymes) work by lowering the activation energy of a reaction so that more reactants can be converted to products. They are not used up themselves in the reaction and do not affect the equilibrium. They only speed up the rate at which equilibrium is achieved.
- Q:What is the superiority of the catalyst compared to the stoichiometric reagent?
- Activity adjustable: including inhibitors and activator regulation, feedback inhibition regulation, covalent modification and allosteric regulation.
- Q:What are the catalysts?
- The catalyst is a substance that can change the rate of the reaction without changing the standard of the reaction Gibbs free, according to the definition of the International Pure and Applied Chemistry (IUPAC) in 1981, Enthalpy change. This action is called catalysis. The reaction involving the catalyst is a catalytic reaction. The catalyst will induce a chemical reaction to change, causing the chemical reaction to become faster or slower or to undergo a chemical reaction at a lower temperature The catalyst is also known as a catalyst in industry, and the composition, chemical properties and quality of the catalyst itself do not change before and after the reaction;
- Q:What is the catalyst for ethylene addition water? How to play a catalytic role.
- Phosphoric acid or sulfuric acid, 280 to 300 ° C, 7 to 8 MPa
- Q:A substance involved in chemical reflection, but reflects the quality of the material before and after the change, you say it is a catalyst?
- Is a catalyst. Principle and burning black copper wire and ethanol reaction to produce acetaldehyde the same
- Q:The size of △ H in the thermochemical reaction equation is related to the use and unused catalyst
- There is no relationship between the catalyst can only change the reaction rate
- Q:The chemical equation of heating reaction of benzene and hydrogen under the action of catalyst
- C6H6 benzene + 3H2 - (arrow) C6H12 cyclohexane (Ni catalytic heating)
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Amino Trimethylene Phosphonic Acid Liquid And Solid
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products

























