Amino Trimethylene Phosphonic Acid Corrosion Inhibitors
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Product Description:
Amino tris(methylene phosphonic acid) / Amino Trimethylene Phosphonic Acid/ ATMP / 6419-19-8 / C3H12NO9P3
CAS No. 6419-19-8
Molecular Formula: N(CH2PO3H2)3
Molecular weight: 299.05
Structural Formula: 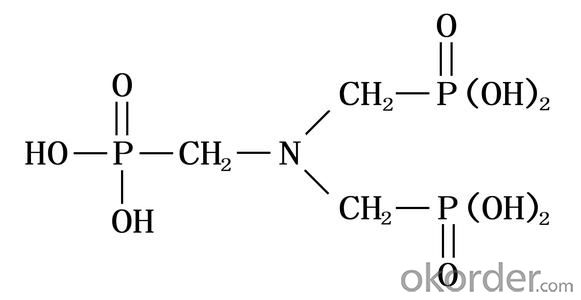
Properties:
ATMP has excellent chelation, low threshold inhibition and lattice distortion ability. It can prevent scale formation, calcium carbonate in particular, in water system. ATMP has good chemical stability and is hard to be hydrolyzed in water system. At high concentration, it has good corrosion inhibition.
ATMP is used in industrial circulating cool water system and oilfield water pipeline in fields of thermal power plant and oil refinery plant. ATMP can decrease scale formation and inhibit corrosion of metal equipment and pipeline. ATMP can be used as chelating agent in woven and dyeing industries and as metal surface treatment agent.
The solid state of ATMP is crystal powder, soluble in water, easily deliquescence, suitable for usage in winter and freezing districts. Because of its high purity, it can be used in woven & dyeing industries and as metal surface treatment agent.
Specification:
| Items | Index | |
|---|---|---|
| Standard | Solid | |
| Appearance | Clear, Colorless to pale yellow aqueous solution | White crystal powder |
| Active acid % | 50.0-51.0 | 95.0min |
| Chloride (as Cl-)% | 1.0 max | 1.0 max |
| pH value (1% solution) | 2.0 max | 2.0 max |
| Fe,mg/L | 10.0max | 20.0max |
| Density (20°C)g/cm3 | 1.31-1.35 | - |
| Colour APHA (Hazen) | 30.0max | - |
Application range&using method:
ATMP is usually used together with other organophosphoric acid, polycarboxylic acid and salt to built all organic alkaline water treatment agent. ATMP can be used in many different circulating cool water system. The recommended dosage is 5-20mg/L. As corrosion inhibitor, The recommended dosage is 20-80mg/L.
Package and Storage:
ATMP liquid: Normally In 30kg or 250kg net Plastic Drum;ATMP solid: 25kg inner liner polyethylene (PE) bag, outer plastic woven bag, or confirmed by clients request.Storage for ten months in room shady and dry place.
Safety Protection:
ATMP is Acidity, Avoid contact with eye and skin, once contacted, flush with water.
Shipping Date: Within 7-10 workdays after receiving your deposit.
Our Service:
Own Lab and joint venture factory.
Superb r&d team;Safety standardization production.
Rich experience in export and strong logistical support.
Good relationship with many large domestic pharmaceutical factory.
Perfect service, perfect supply chain.
- Q:When there is a catalyst in the chemical equation, it is not necessary to match the atoms of the catalyst
- No need, because the catalyst in the chemical reaction before and after the quality of the same
- Q:Is there a catalyst for a chemical reaction?
- There may be many, but some of the catalytic effect of the catalyst is good, and perhaps some of the catalyst has not been found
- Q:What is chemical adsorption and its relationship with heterogeneous catalysis
- The catalytic cycle includes five steps: diffusion, chemical adsorption, surface reaction, desorption and reverse diffusion.The chemical adsorption is an important part of the heterogeneous catalysis process, and the adsorption of the reactants on the catalyst surface,
- Q:Why the catalyst is required to have a large surface area and a rich pore structure
- Chemical reactions are generally contact reaction, of course, the more contact with the faster response, pore structure is to increase the contact area
- Q:If possible can anyone give me information on the active site, substrates, products, and the energy of activation as part of the answer?Responses greatly appreciated! Thankss! 10pts to best answer!
- To make it simple unlike the dude above me...enzymes (biological catalysts) lower the activation energy, which speeds up the reaction. EVERY reaction needs a little boost of energy--the activation energy--and enzymes lower that.
- Q:The greater the chemical adsorption strength, the catalyst activity changes
- If the adsorbent is a reactant, then the better the adsorption capacity of the better catalytic effect; but the catalyst surface of the product will generally have adsorption, if this effect has become very strong, then desorption The process will become difficult, the catalytic effect will decline; the other one, if the adsorption of other substances, such as the reaction may produce a reaction or the catalyst will poison the material, it is greatly detrimental to the catalytic effect. The effect is to be controlled in a suitable optimum range for superior, and preferably to be selectively adsorbed.
- Q:in acid-catalyzed reaction,there are some books show the acid catalyst as H+ and there are some show it as H3O+ .Are they the same?
- Short=yes they are. Long version Traditionally, acids were defined to be compounds that produce H+ ions when dissoved in water (Arrhenius theory). But this definition is limited to acids that can be dissolved in water. Br?nsted-Lowry then formed a definition which states that acids are compounds which donates a protons or H+ ions. If u think about it, a H+ ion is practically a proton. a proton with no electron outer shell is far too reactive to stay in its current state. thus it will form a bond with H2O to form H3O+ Because of this, acid catalysts are supose to be H3O+ instead of H+. But since it is more convinient to use H+, the form of writing H+ remained instead....... and yes...... the scientists were lazy......
- Q:Co and No form a chemical equation for Co2 and No2 under the action of a catalyst
- 2CO + 2NO == N2 + 2CO2
- Q:Why can some catalysts be reused in (chemistry)?
- Because the catalyst in the chemical reaction before and after the quality and chemical properties have not changed, so in the chemical reaction can be reused.
- Q:Chemical Glossary: Catalyst
- The catalyst is a substance that can change the rate of the reaction without changing the standard of the reaction Gibbs free, according to the definition of the International Pure and Applied Chemistry (IUPAC) in 1981, Enthalpy change. This effect is called catalysis. The reaction involving the catalyst is a catalytic reaction.
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Amino Trimethylene Phosphonic Acid Corrosion Inhibitors
- Loading Port:
- Tianjin
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- 6000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products

























