Aluminum Foil with Cryogenic Insulation Paper
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 1 m²
- Supply Capability:
- 100000 m²/month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
The fire retardation glass fiber Cryogenic & Insulation paper is made of superfine glass fiber with special processing. This is usually used with aluminium foil and used for transport containers which store liquid helium, liquid hydrogen, liquid nitrogen,LNG etc.
The superfine glass fiber Cryogenic & Insulation paper's diameter between 0.1-0.6μm and fiber glass filament (diameter between 3-5 μm). According to a certain proportion, the paper is produced under the wet & vacuum forming process to make the paper have the space lattice structure. All the glass fiber is inorganic material, so it has outstanding flame retardant properties. Specifically engineered for use at big temperature range (approaching -273℃—500℃) and also the paper is really stable in the low temperature condition.
Cryogenic Insulation Paper Features:
The main way of heat conduction is by radiation heat exchange in the low and vacuum condition. In order to reduce the radiation heat exchange to meet the good heat insulation effect, by using aluminum foil for reflecting screen and superfine glass fiber insulation paper for spacer, then the multilayer combination of aluminum foil reflective screen and cryogenic & insulation material can build up the efficient thermal barrier against heat transfer in insulated vacuum storage containers. This material can be freely winding, and help the user to reduce the waste of manpower and material waste.
Cryogenic Insulation Paper Application:
Cryogenic liquid (liquid helium, liquid hydrogen, LNG, liquid oxygen, liquid nitrogen, liquid argon, etc.)
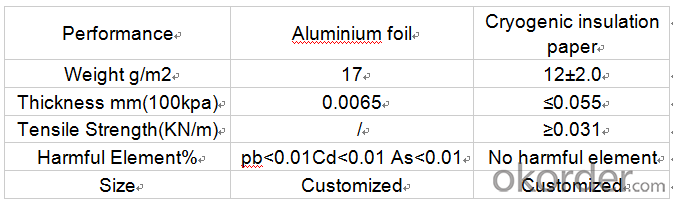
Cryogenic Insulation Paper Specifications
1.light weight
2.good uniformity
3.low thermal conductivity
Technical data:
Q1 What’s the transport method?
A1 FCL delivery goods with wooden pallet; If LCL delivery, must with wooden case; Sometimes need open top, flat rack or bulk cargo.
Q2 What’s the required payment term?
A2 Generally 30% TT as the prepayment, 70% TT before delivery. If need, 100% Irrevocable Letter of Credit or negotiation.
Q3 Which country are our products exported to?
A3 Apart from entire Chinese market, the US, Russia, Japan, Korea, Australia and some Southeast Asian Nations.


- Q:What are the conditions for the registration of large medical equipment exams?
- ? Which doctors professional candidates who have to hold "physician practice certificate." ?????? Working hours as of time for the examination time.
- Q:The first configuration to be reported to the provincial health administrative departments to review the large medical equipment which
- Second, Class A: 1. X-ray positron emission type electronic computer tomography (PET-CT, including positron emission tomography PET) 2. Gamma-ray stereotaxic treatment system (gamma knife) 3. Medical electronic cyclotron acceleration treatment system (MM50) 4. Proton treatment system. 5. Other large medical equipment with a price of RMB 5 million and above.
- Q:Cryogenic cooling circulating pump can be equipped with what equipment
- The equipment is particularly suitable for the need to maintain low temperature, room temperature work under the chemical, biological, physical laboratories, medical and health, chemical industry, food industry, metallurgical industry, universities, scientific research, genetic engineering, Polymer engineering and other laboratory equipment necessary (according to user needs customized large-capacity low-temperature coolant circulating pump).
- Q:but also have better shock performance and anti-extrusion performance, according to the above requirements, the election 20 Medical refrigerated box requirements can be a long time to keep low temperature, dry, but also have better shock resistance and anti-extrusion performance, according to the above requirements, select the best material. (1) the production of cold storage box shell, should be used Cotton Foam Plastic Hardboard (2) cold storage box cooling filler material, should be used Crushed ice cubes low temperature brine foam dry ice (3) cold box inside the shell close to the liner, should be used Soft paper velvet foam leather
- Cooling filler can only choose dry ice. Crushed ice does not meet the "dry, earthquake" requirements; low temperature salt water does not meet the "dry" requirements; foam is nonsense.
- Q:Why is the multi - stage cascade refrigeration cycle of ultra - low temperature equipment
- Summary: multi-stage cascade refrigeration cycle is a representative of the traditional techniques, such as a manual transmission car; single auto-cascade technology represents a more advanced international technology, like automatic car.
- Q:Domestic manufacturers of medical oxygen equipment? Quality must be better, the best brand, seeking recommendations.
- Do the oxygen equipment manufacturers are not many, there are many installation and construction, can really have a specific construction program and the process of the enterprise is very small.
- Q:Need to do a low-temperature equipment, the temperature inside the box to minus 70 degrees, the insulation layer of 100 mm, what is the insulation layer of material for good insulation? Check with polyurethane PU foam is better, but more expensive. With glass fiber cotton insulation afraid of a long time the insulation layer will have condensate and damage the insulation effect, but look at other foreign similar equipment are used glass fiber cotton insulation, may I ask, in the end can use this material to low temperature insulation? There are detailed technical information that I add another 100 points, thank you!
- No, extruded board long-term safe temperature at 70 degrees, extreme temperature at 90 degrees. Extruded board can not, PU can not, more than 180 degrees will be broken down. You only said the original low temperature, did not say high temperature. 180 degrees or more of course can only use glass wool, and no other material optional. You are not afraid to spend money, you can consider the airgel try.
- Q:Medical equipment containing radioactive sources
- Medical equipment containing radioactive sources include: bone density meter, r camera, r knife, cobalt 60 treatment machine, blood irradiator, hospital implantation in the treatment of iodine, iridium, gold and other equipment. Medical radiation devices include: all kinds of X-ray machine, CT machine, C-arm, accelerator, neutron generator. Medical electromagnetic radiation equipment mainly include: B ultrasound, ultraviolet therapy machine, heat cancer machine, high frequency scalpel, microwave acupuncture equipment. Civil radio technology, electromagnetic radiation facilities include: airports, railway stations, station use baggage inspection devices, portable baggage monitor; customs use of radiation devices; radio station launch equipment; meteorological, civil aviation departments use radar, navigation and launch equipment ; Power companies, power plants use substations, switch stations; road bridge dams used in water measuring instrument; Academy of Sciences used in the test, tracer equipment; pressure detection industry used r detection machine. Industrial electromagnetic radiation equipment and nuclear technology application facilities include: steel processing, paper industry, the use of the thickness gauge, moisture measuring instrument; cement, thermal power, coal mining industry, said the use of nuclear, level meter; Gold fineness instrument, the nuclear said; industrial non-destructive testing industry used in the flaw detection machine. Coal, lead, zinc, copper, iron ore, rare earth, niobium / tantalum, zircon and zirconia industry, tin, phosphate industry, aluminum, vanadium and other eleven types of mineral development enterprises will be reported as associated radioactive ore
- Q:Chapter 6 of the management and use of large medical equipment
- Health Bureau: According to the "large-scale medical equipment configuration and use of management approach" (Wei Zhengfa Fa [2004] No. 474) provides that the third batch of large-scale medical equipment management items are announced. Please strictly in accordance with the provisions of large-scale medical equipment management, conscientiously implement the system, and further do a good job in large-scale medical equipment configuration, use and centralized procurement management. January 17, 2013 annex: A large medical equipment management items (third batch) First, the positron emission magnetic resonance imaging system (English referred to as PET-MR, including the integration and split two types) Second, TrueBeam, TrueBeam STX Medical Linear Accelerator Third, Axesse type medical linear accelerator
- Q:How can there be a low temperature cabinet in Hangzhou
- Road outside the medical device street, near the Arab square, ask the passers-by on it
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Aluminum Foil with Cryogenic Insulation Paper
- Loading Port:
- Shanghai
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 1 m²
- Supply Capability:
- 100000 m²/month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Hot Searches
Related keywords