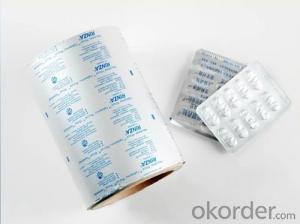
Aluminium Foil Jumbo Roll for Medcine Packaging
- Loading Port:
- China main port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 3 m.t.
- Supply Capability:
- 10000 m.t./month
OKorder Service Pledge
OKorder Financial Service
You Might Also Like
Item specifice
1.Description of Aluminium Foil Jumbo Roll for Medcine Packaging
1.structure:op/printing/AL/vc primer/AL/vc
2.thickness:0.012mm-0.036mm
3.width :60mm-780mm
4.Aolly:8011 H18
5.Usage: pharmaceutical blister packaging,heat seal against PVC ,PVDC
6.adopt ISO9001:2000 and SGS KOSHER MSDS
7:can be printed eight colors
2.Why you want to choose us?
We've been specialized in aluminium foil for more than ten years, we know this product very well, we know what is good, what is the market price.
3.Specification and Application of Aluminium Foil Jumbo Roll for Medcine Packaging
Product Name | Pharmaceutical Aluminium Blister Foil |
Color | silver, gold(as customer's requirement) |
Alloy | 8011 |
Temper | Half hard |
Thickness | 0.012mm-0.036mm |
Width | 60mm-780mm |
Core ID | 76mm |
quality tolerance | ±5% |
Structures | Primer/AL/VC OP/Printing/AL/VC |
Functions | High obstruction, Heat insulation,Printable,Breakable in slightly |
Certification | ISQ9000,SGS,MSDS,KOSHER |
MOQ | 500KG |
Payment | TT, L/C |
Delivery Time | 30-40 days after advanced payment |
Packing | wooden box/pallet as your request |
Applications | Blister packages for pharmaceutical products such as capsules and tablets,pill,pastille, Small-size food, candy, etc, heat seal against PVC ,PVDC |
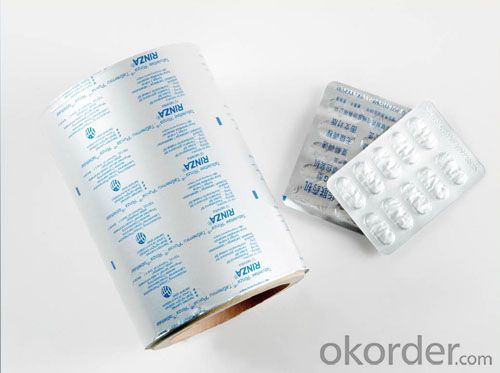
4.Pictures of Aluminium Foil Jumbo Roll for Medcine Packaging

5.FAQ
1) How about your company?
A world class manufacturer & supplier of aluminum coil and alloy blanks. Aluminum production base is comprised of 18 aluminum annealers, 10 coil and foil mills, 4 continuous production lines, 2 hot rolling production line and 3 prepainted lines.
Export 5000 tons per month to Asia, America and Middle East. Always do the best for our clients.
2) Can you guarantee the quality of the products?
We are responsible for the quality of materials to get a long-term cooperation with clients in a reasonable period of time and we are glad to arrange and coordinate any third party inspection for you.
3) What is the delivery time after purchase?
35 day after receiving client’s deposit or correct LC
- Q:What kind of welding rod should be used for aluminum plate, welding and welding?.
- Aluminum plate welding, if it does not take into account the deformation or the objective conditions of the gas, it is recommended to use AC aluminum arc welding machine welding, welding is also commonly used.If considering the deformation and welding method is more simple words, can be used in low temperature aluminum WE53 solid aluminum wire flame welding, the welding is more suitable for super thin aluminum sheet, the thinner the better welding.If it is in the plate, and no argon arc welding machine or manual, you can use the aluminum welding wire welding, aluminum welding wire welding using WEWELDING 555 aluminum electrode welding imports, there is a reference video you can go to see "the use of WE555 aluminum welding wire welding in the Aluminum Alloy"Do not understand, you can continue to ask
- Q:Are the aluminum sheets suitable for manufacturing automotive chassis?
- Yes, aluminum sheets are suitable for manufacturing automotive chassis. Aluminum is lightweight, strong, and resistant to corrosion, making it an ideal material for constructing the chassis of vehicles.
- Q:What is the modulus of elasticity for aluminum sheets?
- The modulus of elasticity for aluminum sheets, also known as Young's modulus, is typically around 69 GPa (gigapascals) or 10 million pounds per square inch (psi). This value represents the measure of the stiffness or rigidity of the material. However, it is important to note that the modulus of elasticity can vary slightly depending on the specific alloy composition and tempering of the aluminum sheet.
- Q:What are the different surface patterns available for aluminum sheets?
- Depending on the desired aesthetic and functional requirements, aluminum sheets can exhibit a range of surface patterns. Some commonly used patterns include: 1. Smooth Finish: This basic pattern offers a flat and polished appearance, ideal for applications that prioritize a clean and sleek look, such as architecture and interior design. 2. Stucco Embossed: Featuring a raised texture resembling stucco, this pattern enhances durability and slip resistance. It is suitable for surfaces like stair treads or ramps that require a non-slip surface. 3. Diamond Tread: This pattern consists of raised diamonds on the surface, providing superior traction and slip resistance. It is commonly used in applications where safety is a concern, such as vehicle running boards or industrial flooring. 4. Hammered Finish: Creating a textured appearance similar to hammered metal, this pattern adds visual interest and can be used to enhance the aesthetics of architectural elements, decorative panels, or furniture. 5. Brushed Finish: Also known as satin finish, this pattern is achieved by brushing the aluminum sheet in a linear direction. It creates a smooth and consistent texture, often used for decorative purposes in applications like signage, wall cladding, or kitchen appliances. 6. Perforated: In this pattern, the aluminum sheet is punctured with small holes or slots in a specific design or pattern. It allows for airflow, light transmission, and sound absorption, making it suitable for applications like ventilation systems, acoustic panels, or decorative screens. These examples illustrate the variety of surface patterns available for aluminum sheets. The choice of pattern depends on the intended use, desired appearance, and functional requirements of the application.
- Q:I heard somewhere that when aluminum is heated, it releases something that is a known cause of Alzheimers. Is this true? I have a homemade cooker made of aluminum and I don't want Alzheimers.
- Aluminium and aluminium compounds are ubiquitous and we consume up to 50mg a day. The fact that aluminium is present isn't necessarily an indication that it is the cause of Alzheimers, and the consensus is that there are a combination of factors at play including genetic factors. However, there has been research which reveals amyloid plaques, which are protein compounds and aluminium and tangled synapses, which are part of the neuronal network. This is leading researchers to suspect that there is a causal link.
- Q:What is the use?
- The three and 3000 series aluminum on behalf of 300330033A21. Also known as China's 3000 series aluminum antirust aluminum production process is more outstanding.3000 series aluminum is made of manganese as main components. The contents of 1.0-1.5 in between. Is a series of anti rust function better. Routine application in air conditioning, ice box, car etc. in the moist environment, price more than 1000 written columns, is a commonly used alloy series.The four and 4000 series aluminum aluminum on behalf of 4A014000 series belongs to the high silicon content series. Usually silicon content between 4.5-6.0%. Belongs to the building material, mechanical parts, forging materials, welding materials; low melting point, good corrosion resistance of the product description: has the characteristics of heat resistance, wear resistanceThe five and 5000 Series 5052.5005.5083.5A05 series.5000 series aluminum plate belongs to the representative of the more commonly used aluminium alloy series, the main elements of magnesium, magnesium content in 3-5%. Also called aluminum magnesium alloy. The main characteristics of low density, high tensile strength, elongation rate is high. In the same area under the weight of the aluminium magnesium alloy is lower than that of other series. It is commonly used in aviation, such as aircraft fuel tank. The application in the conventional industries are more widely. The processing technology for slab continuous casting and rolling, which belongs to the aluminum plate series so can do deep processing. Oxidation belongs to one of the more mature aluminum series in China 5000 series aluminum plate.
- Q:Can aluminum sheets be brushed or satin finished?
- Yes, aluminum sheets can be brushed or satin finished.
- Q:I have a specific design I'd like to cut out of thin sheet metal (aluminum or tin) and I'm wondering how to make it more sturdy. The sheet metal is a bit flimsy. Can I strengthen it by heating it up (butane torch) and cooling it quickly?
- This Site Might Help You. RE: Can you temper aluminum or tin? I have a specific design I'd like to cut out of thin sheet metal (aluminum or tin) and I'm wondering how to make it more sturdy. The sheet metal is a bit flimsy. Can I strengthen it by heating it up (butane torch) and cooling it quickly?
- Q:Are aluminum sheets suitable for pharmaceutical applications?
- Yes, aluminum sheets are suitable for pharmaceutical applications. They are commonly used for packaging pharmaceutical products due to their excellent barrier properties, lightweight nature, and resistance to corrosion. Aluminum sheets help to protect the contents from moisture, oxygen, and light, ensuring the stability and effectiveness of pharmaceutical drugs. Additionally, aluminum sheets can be easily formed into different shapes, making them ideal for creating blister packs, caps, and closures for pharmaceutical packaging.
- Q:Are aluminum sheets resistant to abrasion?
- Yes, aluminum sheets are generally resistant to abrasion due to their hardness and durability.
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
Aluminium Foil Jumbo Roll for Medcine Packaging
- Loading Port:
- China main port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- 3 m.t.
- Supply Capability:
- 10000 m.t./month
OKorder Service Pledge
OKorder Financial Service
Similar products
New products
Hot products
Related keywords