ADAPTOR for PVC
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
Quality Product, Order Online Tracking, Timely Delivery
OKorder Financial Service
Credit Rating, Credit Services, Credit Purchasing
You Might Also Like
Specifications
1.Standard: ISO4422-1996, GB/T10002.1-2006
2.Material: PVC
3.Size: DN63-DN400
4.Connection: socket end with rubber ring
PVC flange socket adaptor
| Material:PVC |
| Size: DN63-DN400 |
| Standard: ISO4422-1996, GB/T10002.1-2006 |
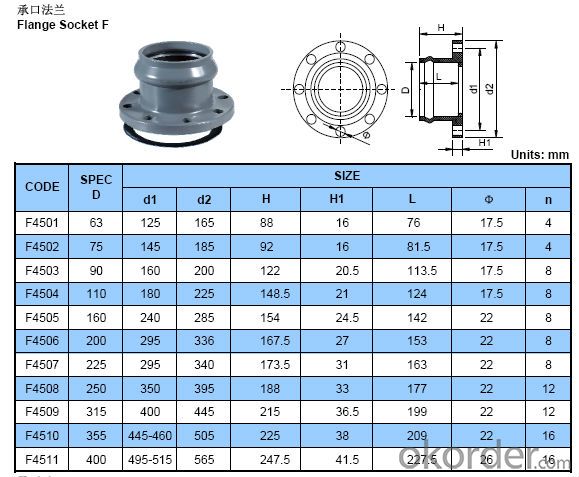
1. Connection: socket end with rubber ring
2. Color: Grey
3. Use: Widely used in water supply and drainage system
- Q:Hi!How would you prepare Iron Pyrites, a.k.a Iron Sulphide, Fool‘s Gold, FeS2, in the lab???
- Iron disulfide (FeS2) is prepared in the lab by heating powdered Iron with Sulfur. The resulting compound is not the mineral Iron pyrite. Iron pyrite is the cubic crystalline form of this compound. It requires special conditions of cooling and pressure for Iron disulfide to form Iron pyrite. Because the natural mineral is very inexpensive and abundant, artificial methods of production are usually not attempted.
- Q:during a period or implantation bleeding, if you pass some small clots, is that due to having low iron?? if not, what are clots and why do some poeple pass them while bleeding??
- idk im anemic i dont know what a clot is what is it?
- Q:been taking iron supplements individual for a couple years now and read some where that multivitamins that contain iron,zinc, potassium can stain teeth? never heard of such a thing. but mostly liquid iron?thanks
- This Site Might Help You. RE: does iron supplements stain teeth? been taking iron supplements individual for a couple years now and read some where that multivitamins that contain iron,zinc, potassium can stain teeth? never heard of such a thing. but mostly liquid iron? thanks
- Q:I‘m doing an essay which requires in depth details about the role of iron in bacteria. i‘ve been searching for a while but can‘t find a place that goes into detail more than saying all things need iron.Any help would be appreciated
- Iron plays the same role in bacteria much as it does in humans (apart from its role in hemoglobin, that is). Iron acts as an enzyme cofactor. Many enzymes in bacteria (and every other life form on Earth) require metal ions to be active. We call these specific ions co-factors. That is why there is a whole field of study on proteins called siderophores. These iron scavenging proteins can rip iron away from the environment.whether that be a host organisms (like us) or some mud puddle. Iron, in some environemnts, can actually be a limiting nutrient for growth. For more information, I would google (or pedia search) the terms siderophore, lactoferrin, and transferrin. I hope this helps! Good luck!
- Q:Iron absorption in achlorhydria?PPIs and H2RAs increase gastric pH by blocking acid secretions and making the overall gastric contents more alkaline. Subsequently an individual who ate foods rich in iron would not absorb that iron because the food sources need to be converted from ferric to it‘s ferrous formhaving said that.how would a person with achlorhydria alter iron salts like ferrous gluconate, fumurate, and sulfate?? Thanks!
- It is true that iron absorption is decreased in achlorhydric states, but there are other factors affecting inorganic iron absorption, for example, the intestinal brush border cells also produce ferric reductase, which can convert the insoluble ferric iron to the ferrous form, especially in the duodenum where majority of the absorption occurs. In clinical practice, achlorhydric states and patients with iron deficiency anemia refractory to oral iron supplements are treated with Parenteral Iron supplements.there are I.M and I.V formulations available. So that's always the other option. Just read your additional details this is my understanding of the process. Dietary iron can be taken in two forms, Organic and Inorganic. Organic iron which is contained in heme, from animal meat sources etc are not subject to the above mechanisms. But in the case of inorganic iron, it needs to be made soluble first in order for absorption to take place. So the insoluble ferric form needs to be reduced to the soluble ferrous form this is where the gastric pH comes into picture. Reduction means addition of hydrogens (or removal of oxygen), so in a low gastric pH, there will be an excess of hydrogen ions, and that will help reduction of ferric to ferrous, thus aiding in absorption. This should be explained in detail in a Medical Physiology book like Guyton. Btw, there have been a lot of papers written investigating achlorhydria and iron absorption, try searching for medical journals.try searching for Omeprazole therapy and iron deficiency this was a highly researched topic because proton pump inhibitors are known to cause achlorhydria. If I remember correctly, none of them give a clear cut causal relationship to iron deficiency, although all suggest a correlation. Best Regards
- Q:It seems to get hot. I have tried ironing dress shirts or clothes on several occasions. Usually all cotton dress shirts. No matter how long I iron on them it doesnt work. I pick the shirt up and it looks wrinkled still what in the heck am I doing wrong?
- It is either you are putting not enough or too much pressure on the clothes you are ironing. You may also want to check the setting of your flat iron. You see, the purpose of the control tab in flat iron is for you to adjust the heat level of the iron according to the fabric of your clothes. Say you are ironing cotton then you should set your flat iron on high .
- Q:My iron was 32 on a recent blood test and I looked back and last year at this time it was 146. my hemoglobin and hematocrit are within normal limits and when I got the blood test I had been off my period for about 2 weeks (I have moderate periods that last about 3 days and I only bleed heavy for the first day) should I be concerned? I asked a doctor that I work with and he said because I am a menstuating woman it is ok. I am just worried about the drop from last year to this year. anyone. thanks in advance for your answers.
- Perhaps you need more vitamin C to help absorb more iron. Most nuts contain plenty of iron and liver even more. Red meat is a good source of readily absorbable iron and there is also a reasonable amount of iron in bread, cereals and muesli. Oysters and mussels are also rich in iron. Spinach and broccoli contain some iron as well.
1. Manufacturer Overview |
|
|---|---|
| Location | |
| Year Established | |
| Annual Output Value | |
| Main Markets | |
| Company Certifications | |
2. Manufacturer Certificates |
|
|---|---|
| a) Certification Name | |
| Range | |
| Reference | |
| Validity Period | |
3. Manufacturer Capability |
|
|---|---|
| a)Trade Capacity | |
| Nearest Port | |
| Export Percentage | |
| No.of Employees in Trade Department | |
| Language Spoken: | |
| b)Factory Information | |
| Factory Size: | |
| No. of Production Lines | |
| Contract Manufacturing | |
| Product Price Range | |
Send your message to us
ADAPTOR for PVC
- Loading Port:
- China Main Port
- Payment Terms:
- TT OR LC
- Min Order Qty:
- -
- Supply Capability:
- -
OKorder Service Pledge
Quality Product, Order Online Tracking, Timely Delivery
OKorder Financial Service
Credit Rating, Credit Services, Credit Purchasing
Similar products
New products
Hot products
Related keywords



























